Operating Instructions
130 Pages
Preview
Page 1
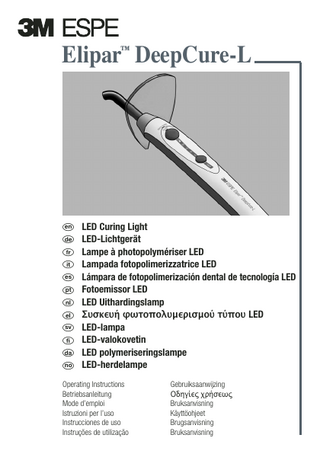
Elipar™ DeepCure-L
en de fr it es pt nl el sv fi da no
LED Curing Light LED-Lichtgerät Lampe à photopolymériser LED Lampada fotopolimerizzatrice LED Lámpara de fotopolimerización dental de tecnología LED Fotoemissor LED LED Uithardingslamp Συσκευή φωτο ολυµερισµού τύ ου LED LED-lampa LED-valokovetin LED polymeriseringslampe LED-herdelampe
Operating Instructions Betriebsanleitung Mode d’emploi Istruzioni per l’uso Instrucciones de uso Instruções de utilização
Gebruiksaanwijzing Οδηγίες χρήσεως Bruksanvisning Käyttöohjeet Brugsanvisning Bruksanvisning
Elipar™ DeepCure-L LED Curing Light
Table of Contents
Page
Safety Instructions
1
Glossary of Symbols
2
Product Description
3
Fields of Application
3
Technical Data Charger Handpiece Charger and Handpiece Transport and Storage Conditions
3 3 3 4 4
Installation of the Unit Factory Settings Initial Steps Charger Light Guide/Handpiece Battery Charging Battery Power Level Display on Handpiece
4 4 4 4 4 4 5
Operation Selection of Exposure Time Activating and Deactivating the Light Inserting and Removing the Light Guide from/into the Handpiece Positioning the Light Guide Testing of Light Intensity Sleep Mode Audible Signals - Handpiece
5 5 6 6 6 6 7 7
Troubleshooting
7
Maintenance and Care Care of the Handpiece Cleaning the Light Guide Clean Handpiece and Glare Shield Storage of the Handpiece during Extended Periods of Non-Use
8 8 8 9 9
Return of Old Electric and Electronic Equipment for Disposal
9
Customer Information Warranty Limitation of Liability
10 10 10
PLEASE NOTE! Prior to installation and start-up of the device, please read these instructions carefully. As with all technical devices, the proper function and safe operation of this device depend on the user’s compliance with the standard safety procedures as well as the specific safety recommendations presented in these Operating Instructions. 1. Use of the device is restricted to trained personnel in accordance with the instructions below. The manufacturer assumes no liability for any damage arising from any other or improper use of this device. 2. The charger must be accessible at all times. Do not use the charger for any use other than the charging of the Elipar™ DeepCure-L handpiece. Disconnect the handpiece from the mains by unplugging the charger from the electrical outlet. Treating patients using the handpiece while it is still connected to the charger is prohibited for safety reasons. Light-curing is possible only if the charger has been disconnected. 3. Use only the charger (AC adapter plug) which is provided with the device. The use of any other charger can result in damage to the battery. 4. CAUTION. Do not stare at source. May be harmful to the eyes. Restrict exposure to the area of the oral cavity in which clinical treatment is intended. Protect patient and user from reflection and intensive scattered light by taking the appropriate precautions, e.g., glare shields, goggles, or coverings. 5. CAUTION! As is the case for all high-intensity lightcuring devices, the high light intensity is accompanied by heat generation on the exposed surface. This heat can result in irreversible damage if there is longer exposure in the proximity of the pulp or soft tissue. The exposure times given in the manufacturer’s instructions must be observed exactly to avoid any such damage. Uninterrupted exposure times of the same tooth surface in excess of 20 seconds and direct contact with oral mucosa or skin must be strictly avoided. Scientists working in this field are in agreement that the irritation caused by heat generated during light curing can be minimized by taking two simple precautions: • Polymerization with external cooling from an air flow • Polymerization at intermittent intervals (e.g., 2 exposures lasting 10 seconds each instead of 1 exposure lasting 20 seconds). 6. Elipar DeepCure-L may be operated only with the supplied light guide or original 3M ESPE Elipar DeepCure-L replacement and accessory light guide. 1
en ENGLISH
Safety Instructions
en ENGLISH
The light guide has to be seen as an applied part. The use of other light guides may result in a reduction or increase in the light intensity. The product’s warranty does not cover any damage resulting from the use of third-party light guides. 7. Condensation resulting from the device being transferred from a cold to a warm environment may be a potential risk. Never begin operating the device until it has reached the ambient temperature. 8. In order to avoid electric shock, do not introduce any objects into the device with the exception of replacement parts handled in accordance with the Operating Instructions. 9. Use only genuine 3M ESPE parts when replacing defective components as directed in these Operating Instructions. The product’s warranty does not cover any damage resulting from the use of third-party replacement parts. 10. Should you have any reason to suspect the safety of the device to be compromised, the device must be taken out of operation and labeled accordingly to prevent third parties from inadvertently using a possibly defective device. Safety may be compromised, e.g., if the device malfunctions or is noticeably damaged. 11. Keep solvents, flammable liquids, and sources of intense heat away from the device as they may damage the plastic housing of the device, the seals, or the operating buttons. 12. Do not operate the device in the proximity of flammable mixtures. 13. Do not allow any cleaning agents to enter the device during cleaning as they could cause an electrical short or a dangerous malfunction. 14. Only service centers authorized by 3M Deutschland GmbH may open the device housing and repair the device. 15. Elipar DeepCure-L must not be used in patients, or by users, with heart pacemaker implants who have been advised to be cautious with regard to their exposure to small electrical devices. 16. Do not use Elipar DeepCure-L in patients with a history of photobiological reactions (including individuals with urticaria solaris or erythropoietic protoporphyria) or who are currently on photosensitizing medication (including 8-methoxypsoralen or dimethylchlorotetracycline). 17. Individuals with a history of cataract surgery may be particularly sensitive to the exposure to light and should be discouraged from Elipar DeepCure-L treatment unless adequate safety measures, such 2
as the use of protective goggles to remove blue light, are undertaken. 18. Individuals with a history of retinal disease should seek advice from their ophthalmologist prior to operating the device. In operating the Elipar DeepCure-L device, this group of individuals must take extreme care and comply with any and all safety precautions (including the use of suitable light-filtering safety goggles). 19. This device has been developed and tested in accordance with the relevant EMC regulations and standards. It is in conformity with legal requirements. Since various factors such as power supply, wiring, and the ambient conditions at the place of operation can affect the EMC properties of the device, the possibility that, under unfavorable conditions, there will be EMC disruptions cannot be completely excluded. If you should notice problems in the operation of this or other devices, move the device to a different location. The EMC manufacturer’s declaration and the recommended separation distances between portable and mobile RF communications equipment and the Elipar DeepCure-L unit are listed in the appendix. 20. Prior to each use of the device ensure that the emitted light intensity is sufficient to safely guarantee polymerization. Check the light guide and the light guide mounting hole to make sure they are clean. If necessary, the light guide mounting hole and the light guide can be cleaned as described in the section “Maintenance and Care” (see also the section “Measurement of Light Intensity”).
Glossary of Symbols Follow instructions for use Attention, consult accompanying documents Type B Equipment Protection against electric shock Protection Class II - double insulated Use in closed spaces only 93/42/EEC Regulatory Compliance Mark for Australia and New Zealand Battery power level Icon to identify electric and electronic devices. The device must be collected and disposed of separately
Light guide Handpiece
en ENGLISH
Glare shield
so that the battery is charged (cf. “Battery Power Level Display on Handpiece”). The device is shipped with a light guide with 10 mm diameter. It is not permissible to use the light guides of other devices. The handpiece is equipped with a sleep mode to minimize the device’s energy consumption. The handpiece switches to sleep mode if it is not used for a period of about 5 minutes or the incorrect charging voltage is detected. The charger uses a maximum of 0.2 W when ready for operation. These Operating Instructions should not be discarded for the duration of use of the device.
Fields of Application Product Description Elipar DeepCure-L is a high-performance LED light source for the polymerization of light-curing dental materials. The device has two components: a wireless handpiece with a built-in battery which can be replaced by 3M ESPE customer care and a charger. The device is a medical electrical device in accordance with IEC 60601-1 and is available as a tabletop device. Wall-mounting is not possible. In comparison with conventional light-curing devices, Elipar DeepCure-L features excellent beam collimation and a uniform beam profile, directing more of the light energy to the restoration being polymerized and producing a deep, uniform, and complete curing of the restoration. The light source is a high-performance light diode (LED). The beam emerging from the device covers the light wavelength range of 430 to 480 nm relevant, for instance, for camphor quinone products and is suitable for use with the majority of light-curing dental materials, including materials for fillings, liners, core build-ups, fissure sealings, temporary restorations, and cements for indirect restorations. See the manufacturer’s information for the exposure time required for the specific dental material. Settable exposure times: • 5, 10, 15, 20 sec • Continuous mode (120 sec) • Tack-cure mode (1 sec) Place the handpiece on a flat surface when not in use. The handpiece can be connected to the charger between applications so that the battery is charged. The handpiece must be connected to the charger at the latest when the battery power level display glows red steadily
• Polymerization of light-curing dental materials with photo initiator for the wavelength range 430-480 nm. - Though the majority of light-curing dental materials are responsive in this range of wavelengths, you may wish to contact the manufacturer of the material in question.
Technical Data Charger Operating voltage:
100-240 V 50/60 Hz
Nominal consumption: 0.2 A max Dimensions without country-specific adapter: Length: 65 mm Width: 40 mm Depth: 31 mm Weight: 75 g Classification: Protection class II, Manufacturer: Click Technology Co., Ltd. Model: CPS 008050100 Handpiece Power supply:
Lithium-ion battery, nominal voltage 3.7 V, capacity 2300 mAh
Utilizable wavelength range: 430-480 nm Wavelength peak: 444-452 nm Light intensity (between 400 and 515 nm): 1470 mW/cm2 -10%/+20% (independent of battery power level) Light emission area: 60-65 mm2 (optically active) 3
Initial Steps Charger 왘 Select the plug adapter specific to the country and
place it on the charger.
앴 앶 앶 앶
Intermittent operation: The device has been designed solely for short-term operation. Typical operating time at room temperature (23 °C): 7 min, at 40 °C ambient temperature: 1 min on, 15 min off (cooling-off period) Total exposure time with new, fully charged battery: Typically 120 min Dimensions: Diameter: 28 mm Length: 270 mm Weight: 180 g (incl. light guide)
Plug adapter
Typ B
Charger and Handpiece Time to charge empty battery: Approx. 2 h Operating temperature: 10 °C up to 40 °C / 59 °F up to 104 °F Relative humidity: 30% up to 75% Atmospheric pressure: 700 hPa up to 1060 hPa Charger
Transport and Storage Conditions: Ambient temperature range: -20 °C up to +40 °C / -4 °F up to +104 °F –20°C –4°F
Relative humidity:
+40°C +104°F
30% up to 75% 75% max.
Light Guide/Handpiece 30%
Atmospheric pressure:
왘 Place the glare shield on the front of the device.
700 hPa up to 1060 hPa 1060
700
hPa
Subject to technical modification without prior notice.
왘 Autoclave the light guide prior to first use. 왘 Then attach the light guide to the handpiece until it
noticeably locks into place (see Section “Removing and Inserting the Light Guide from/into the Handpiece”). 왘 If the device malfunctions, insert the charger plug
into the charging socket of the handpiece. The device will reset itself and can then be used again.
Installation of the Unit Factory Settings The factory settings of the device are as follows: • 10 sec exposure time 4
Battery Charging 왘 The device contains a powerful lithium-ion recharge-
able battery. This type of battery does not have any
en ENGLISH
memory effect and can therefore be recharged at any time by inserting the charger plug into the charging socket of the handpiece (see the section “Battery Power Level Display on Handpiece”).
Operation Selection of Exposure Time
왘 Before using the handpiece for the first time, connect
it to the charger for a period of about 2 hours so that the new battery is completely charged for the first time.
START button
The green status light on the handpiece blinks while the battery is charging. The green status light glows steadily when the device is fully charged. As a safety precaution, light-curing is not possible while the device is charging.
{
LED showing exposure times
TIME button
앶 Status LED 앶앶 앶앶 앶 앴앶
Exposure time options: 5, 10, 15, 20 sec, continuous mode (120 sec), tack-cure mode (1 sec). 왘 See the instructions for use for the specific dental material when selecting the exposure time. 왘 The indicated exposure times assume that the light guide is held at the exact position over the material being polymerized. 왘 If the distance between the light guide and the restoration is increased, the exposure time must be adjusted accordingly because the light intensity weakens (see graph). 100
Battery Power Level Display on Handpiece Operating status Handpiece without Charger connected charger Steady green light
Handpiece ready for operation
Charging has been completed
Flashes green
––
Battery is being charged
Steady red light
Low battery charge Problem during charging
Flashes red
Battery fully discharged, exposure cycle will be completed or, if in continuous mode, stopped
Problem in charging, battery is defective or cannot be charged
90 Light intensity (%)
Status LED
80 70 60 50
0
1
2
3
4 5 6 7 Distance (mm)
8
9 10
Select the exposure time by pressing the TIME button. - The selected exposure time is indicated by the 4 green LEDs. - Each time the button is briefly pressed, the setting advances to the next (higher) value. All 4 green LEDs will be turned on for a setting of 20 sec. Pressing the button again will turn off all of the LEDs and enable the continuous mode. - The display advances through the available settings if the button is kept depressed. 5
- While exposure is ongoing, the button for selection of the exposure time is inactive. Activating and Deactivating the Light 왘 Briefly press the START button; the light will turn on. - The LEDs first indicate the set exposure time; all 4 LEDs light up for 20 sec. Every 5 sec, as the time runs down, the LEDs will turn off one at a time; at 15 sec remaining time, 3 LEDs will still be on, at 10 sec remaining time 2 LEDs, etc. - The LEDs do not come on at all in continuous mode; an audible signal is emitted every 10 seconds. 왘 If desired, the light can be turned off by pressing the START button again before the exposure time is over. 왘 Holding down the START button activates the tackcure mode: the device emits a single short light pulse which enables the defined curing of Protemp™ Crown temporary restorations or a light-curing cement excess (e.g., RelyX™ Unicem) to enable easy removal. Inserting and Removing the Light Guide from/into the Handpiece 왘 Attach the light guide to the handpiece by inserting it with a slight rotation until it clicks firmly into place and there are no gaps between the neck of the light guide and the handpiece (see picture). 왘 Remove the light guide from the handpiece by pulling it towards the front.
Positioning the Light Guide 왘 Rotate the light guide into the desired position for polymerization. 왘 To make full use of the light intensity provided, place the light guide as close to the filling as possible. Avoid directly contacting the filling material. - Keep the light guide clean at all times to obtain full light intensity. - Damaged light guides substantially reduce the light intensity and must be replaced immediately! Sharp edges may cause serious injury! 6
Testing of Light Intensity Depending on the layer thickness of the filling material being cured, it is possible to check the function of the curing light using curing disks for composites: 왘 Place the curing disk on a mixing pad. 왘 Fill your preferred composite into a bore of the curing
disk at least twice as deep as the recommended layer thickness of your composite. 왘 Cure the composite in the curing disk for the time
recommended by the composite manufacturer. 왘 Scrape off soft material from the bottom of the cured
material with a plastic spatula. 왘 The solid thickness of the cured material in the curing
disk divided by two is the layer thickness which can be properly cured. 왘 If you have doubts about the correct function of your
curing light, clean the light guide and the protection glass in the light guide mounting hole and ensure proper fit of the light guide in the handpiece and repeat the intensity test. 왘 If you have still doubts about the correct function of
your curing light, contact 3M ESPE service. The curing disk must only be used to test the function of the curing light! For clinical depth of cure, please refer to the Instructions for Use of the filling material.
Commonly available devices for measuring light intensity can also be used; their measurement values should not be regarded as absolute values. When such devices are used, we recommend recording the intensity of the curing light before its first use and measuring it again at regular intervals so that any decrease in light intensity can be detected. The light intensity testing unit in an Elipar DeepCure-S base station has a device for measuring intensity on a percentage basis.
The handpiece begins charging automatically when connected to the charger (green status light blinks) if the battery requires charging. If the charging voltage is not correct (e.g., there is dirt on the contacts in the charger socket or the charger), the handpiece goes into sleep mode. If the handpiece is not connected to the charger and is not used for a period of about 5 minutes, it also goes into sleep mode. In this operation mode, all of the displays and signals of the handpiece are turned off so that power consumption is reduced to a minimum. To terminate the sleep mode, press the START button. - The sleep mode termination signal (two short audible signals) is emitted, indicating that the handpiece is ready for operation; the handpiece displays the latest selected exposure mode and time settings. Audible Signals - Handpiece An audible signal is emitted • every time a button is pressed, • every time the light is turned ON, • 1 time after 5 sec exposure time, 2 times after 10 sec, 3 times after 15 sec. Exception: in continuous mode, an audible signal is emitted every 10 seconds.
en ENGLISH
Sleep Mode
Troubleshooting Error
Cause 왘 Solution
The status display on the handpiece glows red steadily.
The remaining battery charge is adequate only for typical 5 10-sec exposure cycles. 왘 Connect the handpiece to the charger and recharge the battery.
The status display of The battery lacks sufficient the handpiece flashes charge. red. 왘 Connect the handpiece to The ongoing exposure the charger and recharge is interrupted (light off the battery. signal is emitted) followed by a 2 secerror signal; the handpiece switches to “sleep” mode and resists further activation. The status light on the Charging problem. handpiece blinks red The battery is defective or at whenever the handthe end of its useful life. piece is connected to 왘 Contact 3M ESPE Service. the charger.
Two audible signals are emitted • every time the sleep mode is terminated by pressing the START button, • every time the light is turned OFF.
The handpiece has not been used for a long time and now it cannot be turned on.
A 2-sec error signal is emitted, if
There is not enough charge in the battery to turn on the handpiece. Connect the handpiece to the charger and recharge the battery.
• the handpiece overheats, • the battery lacks sufficient charge. The audible signals from the handpiece can be turned off (except for the 2-sec error signal). Follow these instructions to turn them off. Plug the charger into a functioning power outlet, then press and hold down the TIME and START buttons at the same time with one hand. With the other hand, connect the charging cable from the power supply to the charging socket of the handpiece. An audible signal confirms that there has been a change from “Audible signals activated” to “Audible signals deactivated”. Release the two buttons and disconnect the handpiece from the charger. Repeat the above procedure to activate the audible signals.
The handpiece does not respond to the pressing of either button.
Software crash possible. 왘 Plug the included charger
into an outlet and connect it to the handpiece. This causes the light-curing device to reset itself.
While the handpiece is The handpiece is connected connected to the to the charger. Light-curing charger, pressing the is prevented as a safety START button will not precaution. start the light emission. 왘 Disconnect the charger from the handpiece and restart the light emission. 7
Error
Cause 왘 Solution
The light emission does not start when the START button is pressed; an error signal sounds for 2 sec.
The information about intermittent operation under the header “Technical Data Handpiece” has not been observed. The handpiece has become overheated in the course of use. The handpiece can be used again once it has cooled down.
Maintenance and Care The Elipar DeepCure-L device is maintenance-free. No periodic maintenance is required. See the information contained in this chapter to secure problem-free operation. Care of the Handpiece 왘 Use only the charger included with the product. The
use of other chargers may damage the battery cells or result in inadequate charge. Do not immerse the handpiece in water or incinerate. Please also observe the chapter on “Safety”.
왘 Allow the handpiece to
cool for 3 minutes, then start the next exposure by pressing the START button. During light emission in continuous mode, an error signal sounds for 2 sec, the emission is stopped, and the handpiece changes to sleep mode.
The information about intermittent operation under the header “Technical Data Handpiece” has not been observed. The handpiece has become overheated in the course of use. The handpiece can be used again once it has cooled down. 왘 Allow the handpiece to
cool for 3 minutes, then start the next exposure by pressing the START button. The light intensity is too low.
The dental material does not cure completely.
왘 Clean the light guide and
the protecting glass in the light guide mounting hole (please refer to “Cleaning the Light Guide”). 왘 Clean the light guide and
the protecting glass in the light guide mounting hole (please refer to “Cleaning the Light Guide”). 왘 Check to see that the
correct light guide has been attached. The light guide cannot be attached to the handpiece. 8
왘 The light guide is not
designed for use with the Elipar DeepCure-L.
Cleaning the Light Guide Clean and disinfect the light guide before every use. The light guide is not sterile when delivered and must be autoclaved before being used for the first time. Material Resistance Make sure that the cleaning and disinfectant agents you have chosen do not contain any of the following materials: • Organic, mineral, and oxidizing acids (minimum acceptable pH value 5.5) • Bases (maximum acceptable pH value 8.5) • Oxidation agents (e.g., hydrogen peroxide) • Halogens (chlorine, iodine, bromide) • Aromatic/halogenized hydrocarbons Please check the manufacturer’s information about the cleaning and disinfecting agents. The light guide must not be exposed to temperatures higher than 134° C (273° F). The light guide has been tested for up to 500 sterilization cycles. Pre-Treatment The pre-treatment must be carried out before either automatic or manual cleaning and disinfecting. 왘 Immediately after using (within a maximum of
2 hours), remove gross contaminations from the light guide. 왘 Rinse the light guide off thoroughly (at least
10 seconds) under running water or use a suitable disinfectant solution without any aldehyde (disinfectant should not contain any aldehyde to prevent blood from becoming fixed).
contaminations. Adhering polymerized composite should be removed with alcohol; a plastic spatula may help in removing the material. Do not use any sharp or pointed tools to protect the surface of the light guide from scratching. Manual Cleaning and Disinfection of the Light Guide 왘 Place the light guide for the specified application time
into the solution, making sure that it is completely covered (as needed, using ultrasonic support or careful brushing with a soft brush). A neutral enzymatic cleaning agent is recommended (e.g., Cidezyme/ Enzol from Johnson & Johnson). 왘 Remove the light guide from the solution and rinse
thoroughly (at least 10 seconds) in water with low germ count. 왘 To disinfect, place the cleaned light guide for the
specified application time into the solution, making sure that it is completely covered. Disinfectants containing o-phthalaldehyde are recommended (e.g., Cidex OPA from Johnson & Johnson). 왘 Remove the light guide from the solution and rinse
thoroughly (at least 10 seconds) in water with low germ count.
Clean Handpiece and Glare Shield Clean all components with a soft cloth and, if necessary, a mild cleaning agent (e.g., dish-washing detergent). Solvents or abrasive cleaners can damage the components. Cleaning agents must not enter the device. 왘 To disinfect all components, spray the disinfectant
on a towel and use it to disinfect the device. Do not spray the disinfectant directly on the handpiece. - Disinfection agents must not enter the device! 왘 Dry residual disinfectants with a soft and fluff-free
cloth as they damage the plastic components. 왘 Make sure that disinfectants do not come into contact
with the charging socket on the handpiece because this could impair proper charging. If necessary, ask the manufacturer of the disinfectant if its constant use will damage plastic surfaces. Clean the protection glass with a soft and fluff-free cloth. Beware of scratches! Storage of the Handpiece during Extended Periods of Non-Use 왘 If the handpiece will not be used for a number of
Automatic Cleaning/Disinfection (Disinfector/CDD (Cleaning and Disinfection Device))
weeks - e.g., during vacation - charge the battery beforehand or connect the handpiece to the charger for this time. A safety switch within the battery prevents a total discharge. Discharged or nearly discharged batteries must be recharged as soon as possible.
Alternatively, cleaning and disinfecting can be conducted automatically. Information about validated procedures can be obtained from 3M Deutschland GmbH.
Return of Old Electric and Electronic Equipment for Disposal
왘 Dry the light guide with a clean cloth. 왘 Check the light guide (see section “Check”).
Sterilization Effective cleaning and disinfection are absolutely essential requirements for effective sterilization. Only steam sterilization is approved as a sterilization procedure: - Maximum sterilization temperature 134° C (273° F) - Sterilization time (exposure time at sterilization temperature) at least 20 min at 121° C (250° F) or at least 3 min at 132° C (270° F) /134° C (273° F) Check Before using the light guide again, check it for damaged surfaces, discoloration, and contamination; do not use damaged light guides. If the light guide is still contaminated, repeat the cleaning and disinfection.
1. Collection Users of electric and electronic equipment are required to collect their old equipment separately from other waste in accordance with the regulations of the specific country. Old electric and electronic equipment must not be disposed of with unsorted household waste. This separate collection is a prerequisite for recycling and reprocessing as an important method for preserving environmental resources. 2. Return and Collection Systems When your Elipar DeepCure-L is no longer usable, do not dispose of the device with household waste. 3M Deutschland GmbH has set up special disposal facilities to handle the equipment. Details about the procedure for the specific country can be obtained from the pertinent 3M subsidiary. 9
en ENGLISH
왘 Use a soft brush or a soft cloth to manually remove
3. Removing the Battery for Disposal To dispose of the battery, disconnect the Elipar LED curing light from the charger, remove the two screws on each side of the charging socket and push the lower half of the housing towards the back, away from the upper half of the housing. Use a suitable tool to cut the connecting wires between the battery and the circuit board and remove the battery for disposal in accordance with 1. and 2. 4. Meaning of the Symbols The EU Directive prohibits the disposal of any electric or electronic devices marked with these symbols in combination with household waste.
Customer Information No person is authorized to provide any information that deviates from the information provided in this instruction sheet. Warranty 3M Deutschland GmbH warrants this product will be free from defects in material and manufacture. 3M Deutschland GmbH MAKES NO OTHER WARRANTIES INCLUDING ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. User is responsible for determining the suitability of the product for user’s application. If this product is defective within the warranty period, your exclusive remedy and 3M Deutschland GmbH’s sole obligation shall be repair or replacement of the 3M Deutschland GmbH product. Limitation of Liability Except where prohibited by law, 3M Deutschland GmbH will not be liable for any loss or damage arising from this product, whether direct, indirect, special, incidental or consequential, regardless of the theory asserted, including warranty, contract, negligence, or strict liability.
Information valid as of October 2014 10
Guidance and manufacturer’s declaration – electromagnetic emission The Elipar DeepCure-L is intended for use in the electromagnetic environment specified below. The customer or the user of the Elipar DeepCure-L should assure that it is used in such an environment. Emissions test
Compliance
Electromagnetic environment - guidance
Group 1
The Elipar DeepCure-L uses RF energy only for its internal function. Therefor, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment.
RF emissions CISPR 11 RF emissions Class B CISPR 11 Harmonic emissions Class A IEC 61000-3-2 Voltage fluctuations / flicker emissions
The Elipar DeepCure-L is suitable for use in all establishments, including domestic establishments and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes.
Complies IEC 61000-3-3
125
Guidance and manufacturer’s declaration – electromagnetic immunity The Elipar DeepCure-L is intended for use in the electromagnetic environment specified below. The customer or the user of the Elipar DeepCure-L should assure that it is used in such an environment. IEC 60601 test level
Compliance level
± 6 kV contact
± 6 kV contact
± 8 kV air
± 8 kV air
Electrostatic transient / burst
± 2 kV for power supply lines
± 2 kV for power supply lines
IEC 61000-4-4
± 1 kV for input/output lines
± 1 kV for input/output lines
Surge
± 1 kV differential mode
± 1 kV differential mode
± 2 kV common mode
± 2 kV common mode
< 5 % UT (> 95 % dip in UT) for 0,5 cycle
< 5 % UT (> 95 % dip in UT) for 0,5 cycle
40 % UT (60 % dip in UT) for 5 cycles
40 % UT (60 % dip in UT) for 5 cycles
70 % UT (30 % dip in UT) for 25 cycles
70 % UT (30 % dip in UT) for 25 cycles
< 5 % UT (>95 % dip in UT) for 5 sec
< 5 % UT (> 95 % dip in UT) for 5 sec
3 A/m
3 A/m
Immunity test Electrostatic discharge (ESD) IEC 61000-4-2
IEC 61000-4-5 Voltage dips, short interruptions and voltage variations on power supply input lines IEC 61000-4-11
Power frequency (50/60 Hz) magnetic field
Electromagnetic environment guidance Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30 %. Mains power quality should be that of a typical commercial or hospital environment.
Mains power quality should be that of a typical commercial or hospital environment. Mains power quality should be that of a typical commercial or hospital environment. If the user of the Elipar DeepCure-L requires continued operation during power mains interruptions, it is recommended that the Elipar DeepCure-L be powered froman uninterruptible power supply or a battery.
Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment.
IEC 61000-4-8 NOTE
126
UT is the a. c. mains voltage prior to application of the test level.
Guidance and manufacturer´s declaration – electromagnetic immunity The Elipar DeepCure-L is intended for use in the electromagnetic environment specified below. The customer or the user of the Elipar DeepCure-L should assure that it is used in such an environment. Immunity test
IEC 60601 test level
Compliance level
Electromagnetic environment guidance Portable and mobile RF communications equipment should be used no closer to any part of the Elipar DeepCure-L, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter. Recommended separation distance
Conducted RF
3 Vrms
IEC 61000-4-6
150 kHz to 80 MHz
Conducted RF
3 V/m
IEC 61000-4-3
80 MHz to 2,5 GHz
10 V
3,5 –– d = [–––]√P V1
3 V/m
3,5 –– d = [–––]√P 80 MHz to 800 MHz E1 7 –– d = [–––]√P 800 MHz to 2,5 GHz E1 where p is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in metres (m).b Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey,a should be less than the compliance level in each frequency range.b Interference may occur in the vicinity of equipment marked with the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the Elipar DeepCure-L is used exceeds the applicable RF compliance level above, the Elipar DeepCure-L should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the Elipar DeepCure-L. b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m. 127
Recommended separation distances between portable and mobile RF communications equipment and the Elipar DeepCure-L The Elipar DeepCure-L is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the Elipar DeepCure-L can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the Elipar DeepCure-L as recommended below, according to the maximum output power of the communications equipment Separation distance according to frequency of transmitter m Rated maximum output of transmitter
150 kHz to 80 MHz
80 MHz to 800 MHz
800 MHz to 2,5 GHz
3,5 –– d = [–––]√P V1
3,5 –– d = [–––]√P E1
7 –– d = [–––]√P E1
0,01
0.12
0.40
0.77
0,1
0.37
1.26
2.42
1
1.17
4.00
7.67
10
3.69
12.65
24.24
100
11.67
40.00
76.67
W
For transmitters rated at a maximum output power not listed above the recommended separation distance d in metres (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
128
3M ESPE Customer Care/MSDS Information: U.S.A.1-800-634-2249 and Canada 1-888-363-3685. 3M, ESPE, Elipar, Protemp and RelyX are trademarks of 3M or 3M Deutschland GmbH. Used under license in Canada. © 2014, 3M. All rights reserved.
44000769339/01
3M Deutschland GmbH Dental Products Carl-Schurz-Str. 1 41453 Neuss - Germany