ARJO
Smartsigns Compact SC500 Instructions for Use July 2021
Instructions for Use
148 Pages
Preview
Page 1
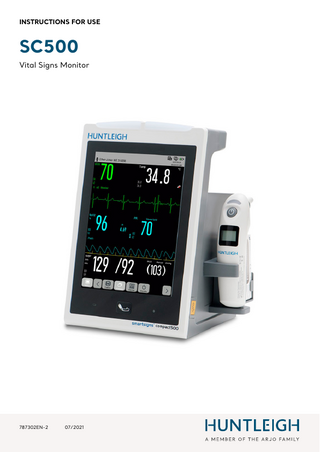
INSTRUCTIONS FOR USE
SC500 Vital Signs Monitor
787302EN-2
07/2021
EN
WARNING To avoid injury, always read this Instructions for use and accompanied documents before using the product. Mandatory to read the Instructions for use.
Please Note: These Instructions For Use apply to the SC500 Vital Signs Monitors (all variants).
© Huntleigh Healthcare Ltd All rights reserved
This symbol signifies that this product complies with the essential requirements of the Medical Device Directive (93/42/EEC) - Medical Device Regulation (EU/2017/745) Manufactured in the UK by Huntleigh Healthcare Ltd. Smartsigns and Huntleigh are registered trademarks of Huntleigh Technology Ltd. 2019. Nellcor OxiMax® is a trademark of Covidien AG © Huntleigh Healthcare Ltd. 2019 As part of the ongoing development programme the company reserves the right to modify specifications and materials without notice 2
EN
Table of Contents 1. Safety Instructions...8 1.1 1.2 1.3
Warnings...8 Infection Control...9 Patient Applied Parts...9
2. Introduction...10 2.1 2.2 2.3 2.4 2.5 2.6
Intended Use...10 Contra-Indications...10 Personal Security Information... 11 Service life... 11 Unpacking / Preliminary Checks... 11 Basic operation and Controls...12
2.6.1
Operator Position...12
3. Product Identification...13 3.1 3.2 3.3 3.4 3.5 3.6
3.7
Front Panel...13 Left Side Panel...14 Right Side Panel...14 Rear Panel...15 Base...16 Display ...17
3.6.1 3.6.2 3.6.3 3.6.4
Waveform Area...17 Parameter Area...17 Upper Menu Bar...18 Lower menu bar...19
Product Labelling...20
4. System Setup ...21 4.1 4.2 4.3
System Connection...21 Patient Connections...21 Handling and Mounting...22
5. Operation ...23 5.1 5.2 5.3 5.4 5.5 5.6
Switching the Unit ON...23 Shortcut keys...23 Standby Mode...23 Touchscreen Lock...23 Switching the Unit OFF...23 Main Menu ...24
5.6.1
General Setup...24
5.6.1.1
Patient Manage...24
5.6.1.2
Alarm Setup...24
5.6.1.3
Review...24
5.6.1.4
Screen Config...24
5.6.1.5
Screens...25
5.6.1.6
Night Mode...26
5.6.1.7
Volume Setup...26
5.6.1.8
Patient Records...26
5.6.1.9
Recorder Setup...26
5.6.1.11 External Printer Setup...26 5.6.1.12 Event Setup...26 5.6.1.13 Parameter Setup...27 5.6.1.14 Configuration Management...28 5.6.1.15 Load Configuration...28
5.6.2
Maintain Menu...28
5.6.2.1
USER Maintain Menu...28
5.6.2.2
Language Setup...28
5.6.2.3
Smoothing...28
5.6.2.4
Network Protocol...29
5.6.2.5
Spot Mode...29
5.6.2.6
Wave Fill Setup...29
5.6.2.7
Time Setup...29
5.6.2.8
Alarm Setup...29
5.6.2.9
Touchscreen Calibrate...29
5.6.2.10 Module Colour...29 5.6.2.11 Units Setup...30 5.6.2.12 Nurse Call Setup...30 5.6.2.13 Module Setup...30
3
EN 5.6.2.14 Monitor Info...30 5.6.2.15 Quick Key Config...30 5.6.2.16 Wave Save...31 5.6.2.17 ECG Calibrate...31 5.6.2.18 NIBP Verify...31 5.6.2.19 Leakage Test...31 5.6.2.20 Format SD Card...31 5.6.2.21 Save Tactics...31 5.6.2.22 Other Setup...32 5.6.2.23 Set User Password...32 5.6.2.24 DEMO...32
6. Configuration Management ...33 6.1 6.2
Overview...33 Managing Configurations...34
6.2.1 6.2.2 6.2.3 6.2.4 6.2.5 6.2.6 6.2.7
Restoring Factory Dafaults...34 Creating and Saving a User Configuration...34 Loading a configuration...35 Deleting a configuration...35 Importing a Configuration from USB...35 Exporting a Configuration to USB ...36 Startup Configuration...36
7. Patient Management ...37 7.1 7.2 7.3 7.4 7.5 7.6
Patient concepts...37 Quick Admit...38 Admitting a Patient...38 Patient Information...39 Managing Patient Records...39 Discharge...41
8. User Interface...42 8.1 8.2 8.3
Standard Display...43 List View...43 Customising the Interface...44
8.3.1 Setting the waveform sweep speed...44 8.3.2 Setting the waveform style...44 8.3.3 Setting the module colour...44 8.3.4 Waveform Fill feature...44 8.3.5 Changing the Displayed modules...44
9. SPOT Check Mode...45 9.1
9.2 9.3
Spot Check Mode – Display Layout...45
9.1.1 Upper Menu Bar...46 9.1.2 Parameter and Waveform Area...46 9.1.3 Spot Check List preview...46 9.1.4 Save Button...46 9.1.5 Manual input Data...46
Spot Check List...47
9.2.1 Spot Check List:-Patient Records...47
Lower Menu Bar...49
9.3.1 9.3.2 9.3.3 9.3.4 9.3.5 9.3.6 9.3.7
Main Menu...49 Admitting a patient (SPOT Check)...49 Patient Group Selection ...49 NIBP Start Stop...49 Score...50 Record...50 Standby...50
10. Alarms...51 10.1 Alarm Classification...51 10.2 Alarm Types...52 10.2.1 10.2.2
Non Latching Alarms ...52 Latching Alarms ...52
10.3 Visual alarm indication ...53 10.4 Alarm tones ...53 10.5 Alarm status messages ...54 10.5.1 Parameter indication...54 10.5.2 Multiple alarms...54
10.6 Setting alarm limits...54
4
10.6.1 Setting alarm limits and priorities...55 10.3.1 Viewing all alarm limits...55 10.6.4 Switching ON alarm limits...57
EN 10.6.5 Switching OFF individual alarms...57 10.6.6 Switching OFF all alarms...57 10.6.8 Pausing the Alarms...58 10.6.9 Defining the Alarm Pause period...58
10.7 Setting the alarm volume...58 10.7.1
Setting the minimum alarm volume...59
10.8 Alarm events...59 10.8.1
Setting alarm events...59
10.9 Alarm System Self-test...59
11. Pulse Rate Setup...60 11.1 Overview...60 11.2 PR Source...60 11.3 PR Alarm Limit Setup...60
12. ECG Monitoring...61 12.1 Precautions...61 12.2 Introduction...61 12.3 Working with Paced Patients...62 12.3.1 12.3.1
Pacemaker Detection – Enabled...62 Pacemaker Detection – Enabled...62
12.4 Monitoring Steps...62
12.4.1 Skin preparation for electrode placement...62 12.4.2 Connecting the ECG Cable...63 12.4.3 ECG lead identification and placement ...63 12.4.3.1 Standard 3 Lead placement (IEC)...63
12.5 ECG Display...64 12.6 ECG Setup...64 12.6.1 12.6.2 12.6.3 12.6.4 12.6.5
Lead Setup...64 Setting the waveform Gain...64 Setting the ECG Sweep Speed ...65 Setting the ECG filter ...65 Notch Filter Setting...65
13. SpO2 Monitoring...66 13.1 Overview...66 13.1.1
Identification of SpO2 technology...66
13.2 Safety...67 13.3 Accuracy verification...68 13.3.1 13.3.2
SpO2 Accuracy...68 PR Accuracy...68
13.4 SpO2 Waveform (Huntleigh)...68 13.5 SpO2 Setup (Huntleigh)...68 13.5.1 Sweep speed...69 13.5.2 Signal IQ...69 13.5.3 NiBP same side...69 13.5.4 Alarm set up...70
13.6 SpO2 Waveform (Nellcor Oximax) ...70 13.7 SpO2 Setup (Nellcor Oximax)...71 13.8 SpO2 Monitoring Steps...72
14. NIBP Monitoring...73 14.1 Overview...73 14.2 Safety...73 14.3 NIBP Connections...74 14.3.1 14.3.2 14.3.3
NiBP Cuff selection and application...75 Limitations of measurement...75 NIBP Display...76
14.4 NIBP Setup...76 14.4.1 14.4.2
Patient Type...77 Measurement Modes...77
14.4.2.1 Manual Mode...77 14.4.2.2 Automatic Mode...77 14.4.2.3 STAT Mode...77
14.4.3 Target Pressure...78 14.4.4 Alarm Limit Setup...78 14.4.5 STAT measurements...78 14.4.6 Reset Module...79 14.4.7 Other Setup (EXTENDED Options)...79 14.4.7.1 Venous Cuff Pressure (mmHg)...79 14.4.7.2 Venipuncture Start...79 14.4.7.3 NiBP Analysis...81
5
EN 14.4.7.4 Dynamic Analysis Trend...82
14.5 Start / Stop Manual BP Measurement...83 14.6 Start / Stop Automatic BP measurement...83
15. Temperature Monitoring...84 15.1 Overview...84 15.2 Safety...84 15.3 Description of the Thermometer IRT10...85
15.3.1 Thermometer Display...85 15.3.2 Pairing the Thermometer and Main Unit...85 15.3.3 Measuring Temperature...86 15.3.4 Wireless Transmission Function...86
15.4 Temperature Display...87 15.4.1
Alarm settings...87
16. Score Calculator...88 16.1 16.2 16.3 16.4 16.5 16.6
Introduction...88 Safety...88 MEWS (Modified Early Warning Score)...88 NEWS2 (National Early Warning Score)...88 Switching between MEWS and NEWS2...89 MEWS System...90
16.6.1 16.6.2
Entering the MEWS calculation screen...90 Saving, reviewing, clearing and printing the MEWS Score...92
16.7 NEWS2 System...93 16.7.1 16.7.2
Entering the NEWS2 calculation screen...94 Saving, reviewing, clearing and printing the NEWS2 Score...95
17. Managing Data...97 17.1 17.2 17.3 17.4
Overview...97 Waveforms...97 Waveform Freeze...98 Review...98
17.4.1 Trend Review...99 17.4.2 NiBP Review...100 17.4.3 Alarm Event Review...100 17.4.4 Waveform Review...102
18. Recorder...103 18.1 Description of Recorder...103 18.2 Loading paper...103 18.3 Recordings...104
18.3.1 Setting up the Recorder...104
18.4 Manual Operation...105 18.5 Automatic Operation...105 18.6 Recorder maintenance...105 18.6.1 Clearing paper jam...105 18.6.2 Cleaning...105
19. External Printing...106 19.1 External printer specification...106 19.2 Report Printing ...106 19.2.1 19.2.2 19.2.3 19.2.4 19.2.5 19.2.6 19.2.7
NiBP List report...107 Real Time Waveform report...107 Wave Review report...108 Alarm event report...109 Trend Table Review Report...110 Trend Graph Review Report... 111 Cancelling a report...112
19.3 Printer Error Messages... 112
20. Extended Functionality...113 20.1 Nurse Call... 113 20.2 Central Monitoring System Interface ... 113 20.3 Formatting SD card... 114
21. Battery...115 21.1 21.2 21.3 21.4
6
Introduction... 115 Battery installation ... 116 Battery optimisation... 116 Battery Recycling... 117
EN
22. Cleaning...118 22.1 22.2 22.3 22.4 22.5 22.6
Cleaning and Disinfecting the Monitor... 118 Cleaning and Disinfecting ECG cables... 119 Cleaning and Disinfecting BP cuffs ... 119 Cleaning and disinfecting SpO2 sensors... 119 Cleaning and Disinfecting Thermometer...120 Precautions...120
23. Maintenance...121 23.1 23.2 23.3 23.4
User maintenance Daily Checks...121 Scheduled Technical maintenance...121 Corrective maintenance...121 Servicing...121
24. Technical Specifications ...122 25. System Alarm Messages...127 25.1 Physiological Alarm Messages ...127 25.2 Technical Alarm Messages...128 25.3 System Prompt Messages...130
26. Default Configuration...131 26.1 General Configuration...131 26.2 Default Alarm Limits...133 26.3 Parameter Default Configuration...134
27. Electromagnetic Compatibility...136 28. Toxic/Hazardous Substances/Elements...140 29. End of Life Disposal...141 30. Warranty & Service...142 Appendix A Accessories...143 Recommended accessories... 143
Appendix B Clinical Evaluation ...146 SpO2 Summary Report ...146 NIBP Summary Report ...146
7
EN
1. Safety Instructions Symbols General Warning Attention, consult this manual. Refer to safety section. Refer to Instructions for Use.
1.1
8
Attention, consult accompanying documents / Instructions for Use.
Warnings WARNING This equipment is for use only by suitably qualified healthcare practitioners.
WARNING Do not use in the presence of flammable gases, pharmaceuticals in aerosols or in oxygen rich environments.
WARNING The monitor is intended only as an adjunct to patient assessment. It must be used in conjunction with clinical signs and symptoms.
WARNING The monitor is a Class 1 device which relies on the earth connection to maintain safety. Ensure it is connected to a suitably earthed AC mains supply.
WARNING Do not use if there is any damage to the unit or its accessories.
WARNING To avoid accidental disconnection, route all cables in a way to prevent a tripping hazard. Secure excess cabling to reduce risk of entanglement or strangulation by patients or personnel.
WARNING Alarm upper and lower limits should be set depending on the patient. Do not rely exclusively on the audible alarm system.
WARNING If this product is connected to another item of electrical equipment, ensure that the system is fully compliant with IEC60601-1.
WARNING Do not use the SC500 in vehicles or in aircraft.
WARNING This equipment must not be modified.
WARNING During defibrillation, the operator should not come into contact with the patient, the monitor or the supporting table; otherwise serious injury or death could result.
WARNING Connect only Items that have been specified as part of the Medical Electrical system or have been specified as being compatible with the Medical Electrical system.
WARNING This unit is not MRI compatible and must not be used in an MRI environment.
WARNING Do not make contact with exposed metal parts and the patient simultaneously.
EN
WARNING PACEMAKER PATIENTS. Rate meters may continue to count the pacemaker rate during occurrences of cardiac arrest or some arrhythmias. Do not rely entirely upon heart rate meter ALARM SIGNALS. Keep pacemaker PATIENTS under close surveillance. See this manual for disclosure of the pacemaker pulse rejection capability of this instrument
WARNING This product contains sensitive electronics, therefore, strong radio frequency fields could possibly interfere with it. This may be indicated as a disturbance on the physiological waveform. We recommend that the source of interference is identified and eliminated.
WARNING Mains isolation is achieved by disconnecting the mains connector to the rear of the SC500 or unplugging from the mains supply
WARNING Do not position the Medical Equipment such that it makes it difficult to disconnect the supply.
WARNING If a loss of power poses an unacceptable risk to the patient the SC500 must be connected to an appropriate mains power source.
WARNING The internal battery must be used if the integrity of the protective earth conductor or protective earthing system is in doubt.
CAUTION The SC500 is restricted to single patient use.
CAUTION Use only recommended accessories listed in this manual.
CAUTION Do not expose to excessive heat, including prolonged exposure to sunlight.
NOTE After defibrillation, the electrocardiogram (ECG) waveform will recover within 5s, other parameters will recover within 10s
NOTE This IFU is based on the maximum configuration. Some content and displays may not be applicable to your monitor.
1.2
Infection Control
The system uses a mixture of reusable and single use accessories, refer to section 24 for specific details.
1.3
Patient Applied Parts
As defined in IEC60601-1:2005, the patient applied parts of the Smartsigns® Compact 500 Vital Signs Monitor are:• •
ECG Cable Blood Pressure cuff
• •
SpO2 Sensor Temperature sensor
9
EN
2. Introduction The Huntleigh Smartsigns® SC500 is a high performance Vital Signs monitor which is capable of performing short, medium and long term monitoring of Adult, Paediatric and Neonate patients. The SC500 can monitor the following parameters: • • • •
2.1
ECG Oxygen Saturation Non-invasive Blood Pressure Body Temperature (Tympanic measurement)
Intended Use
The SC500 Vital Signs Monitor is indicated for use by trained healthcare professionals for the monitoring of Adult, Paediatric and Neonate physiological vital signs. They can be used in a variety of low and mid acuity healthcare settings. The SC500 Patient Monitor provides the following functionality: • • • •
2.2
ECG 3 Lead Blood pressure – non-invasive measurements Oxygen saturation Body temperature (Tympanic Measurement)
Contra-Indications
The device is not intended for use outside a Healthcare facility. No absolute contraindications exist for ECG monitoring, however, clinicians should be aware of situations developing as a result of certain allergies caused by sensitivity to adhesives used to fix electrodes onto the skin. Pulse oximetry is contra indicated for use on active patients or for prolonged use. To prevent tissue damage, sensor position should be checked every 2 hours ( as indicated by circulatory status and or skin integrity) and repositioned every 4 hours. When using wrap type of sensors, do not apply tape to close the sensor, venous pulsation may compromise measurements. Prolonged use and frequent BP determination can lead to venous pooling and or congestion, avoid continuous long term measurements on high risk patients.
10
EN
2.3
Personal Security Information
The SC500 operates in an environment where personal and sensitive data is available, whilst the system offers various levels of access, it is the responsibility of the institution to develop and implement appropriate security measures to comply with local regulations and safeguard personal data.
2.4
Service life
This has been defined as the minimum time period during which the device is expected to remain safe and suitable to meet its intended use, and all risk control measures remain effective. Huntleigh Healthcare Ltd’s commitment is that the expected service life for this Device has been defined as 7 years.
2.5
Unpacking / Preliminary Checks
We recommend that a thorough visual inspection is made immediately the unit is received. Should any damage be evident or any parts missing, ensure that Huntleigh Healthcare Ltd is informed at once. Each configuration is supplied with a patient group specific (ADU, PED or NEO) starter pack of accessories, these include: Each base configuration is supplied with: 1 x NiBP cuff set (3) - ADU, PED or NEO 1 x NiBP Hose (3M) SC500 Base 1 x Huntleigh SpO2 sensor - ADU, PED or NEO configuration 1 x Huntleigh SpO2 Interface Cable(**) 1 x Grounding cable 1 x Power cord 1 x Instructions for use ECG Supplied with: Option 1 1 x 3 Way ECG cable (IEC) - ADU / PED Or 1 x 3 Way Yolk with 1 x pack of pre wired NEO electrodes. Nellcor Oximax SpO2 Supplied with Option 2 1 x Nellcor SPO2 Interface cable 1 x Nellcor SpO2 sensor - ADU, PED or NEO Tympanic Temperature Supplied with: Option 3 1 x Tympanic thermometer (wireless) 1 x Pack of 20 Thermometer probe covers Printer Supplied with: Option 4 Integrated recorder 1 x Roll paper (**) Huntleigh SPO2 Interface cable is provided when the PED or NEO patient groups are specified.
11
EN
2.6
Basic operation and Controls
System management and controls are affected through a hierarchical menu structure with three levels of access control - Basic, Advanced and Engineer. Each level provides access to certain features and functionality described in the table below: Access level
Description
Functionality
Access to Protection patient records method
Basic
Normal use
User interface and controls only
No
None
Advanced
User maintenance
Full system administration
Yes
Password *
Engineer
Factory maintenance
Engineering functionality – establishing and uploading specific profiles, adjustment of local default settings, system calibration etc.
No
Password *
* Refer to your technical support department for password information.
System
Access level
Basic
Adv
Eng
System menu structure
The flexibility of the system means that the operator can access the same control from different areas of the system, for example, menus and system sub menu control can be accessed through: • Physiological waveform • Numerical area of the screen • Soft keys
2.6.1 Operator Position The operator should be positioned in-front of the SC500 for optimal viewing of the display and touchscreen operation.
12
EN
3. Product Identification WARNING Safety and performance can only be assured when the system is used in conjunction with the correct type of accessory. Do not attempt to use any accessory other than those supplied or recommended by Huntleigh.
3.1
Front Panel 6
2
1
3 4 1
Touchscreen
2
Wireless Infrared thermometer (IRT10)
3
4
5
AC power indicator ◊ On: The monitor is connected to AC power. ◊ Off: The monitor is not connected to AC power. Battery indicator ◊ On: The monitor is equipped with a battery and is connected to AC power. ◊ Off: battery is fully charged, is not installed or malfunction. ◊ Flashing: The monitor is running on battery power.
5
NIBP Start/Stop Button
6
Alarm indicator (left indicator is for physiological alarm and the right indicator is for technical alarm). 13
EN
3.2
Left Side Panel
1
7
1
Carry Handle
2
Internal thermal recorder
3
ECG cable connector
4
NIBP connector
5
HUNTLEIGH SpO2 connector*
6
Recorder power indicator
7
Recorder error indicator
6 2 3 4 5 * Depending on model/options purchased.
3.3
Right Side Panel
2 1
14
3
1
On/Off
2
Wireless Infrared thermometer (IRT10) Holder
3
Thermometer probe cover holder
EN
3.4
Rear Panel
9 8 7 6
1
5
2 3
4
1
Mains socket
6
USB Port x 2
2
Equipotential earth point
7
Ethernet port
3
IEC Mains cable retaining clip
8
IV Pole attachment
4
Product identification label
9
Loudspeaker
5
Multifunctional Interface * WARNING If this product is connected to another item of electrical equipment, ensure that the system is fully compliant with IEC60601-1. WARNING The ear thermometer supplied with this monitor can only communicate with an appropriate Huntleigh monitor.
NOTE Lift the IEC Mains cable retaining clip, fully insert the connector before lowering the clip fully over the connector.
15
EN
3.5
Base 1
2
16
1
Battery Compartment
2
Roll stand adapter plate fixing screw location
EN
3.6
Display
This monitor uses a backlit colour LCD display with integrated touch screen. Physiological parameters, waveforms, alarm messages, date and time, network connection status, battery level and other messages are displayed simultaneously. The screen is divided into four areas: • • • •
Waveform area Parameter area Upper menu bar Lower menu bar
Waveform Area
Parameter Area
Upper Menu Bar
Lower Menu Bar
3.6.1 Waveform Area • •
2 waveforms can be displayed, along with the respective identification Touch a waveform to display the corresponding setup menu.
3.6.2 Parameter Area • • •
Displays measured parameters Parameters are displayed in the same colour as their waveform Touch a parameter to display the corresponding setup menu
17
EN
3.6.3 Upper Menu Bar
1
4
2
5
3
7
6
1
Physiological Alarm Status
2
Technical Alarm Menu
3
Alarm Status identification
4
Patient group identification
5
Pacer Status identification
6
Patient Name Monitor setup area: USB drive status, SD card status, Central Monitoring System (CMS) status, Battery status, Date and Time
7
CMS enabled
CMS disabled
SD card inserted
SD card not inserted
Battery status
USB flash drive connected
The UPPER menu bar includes the following indicators, from left to right: 1. Physiological alarm status: Displays the active physiological alarm messages. 2. Technical alarm message: Displays technical alarm status. 3. Alarm Status indicator: Indicates the status of the Auditory alarm system •
Alarm Pause
•
Alarm Off Patient data:-
•
Patient name
•
Patient Group
•
Pacemaker Status. Date and Time.
4.
5.
18
EN
3.6.4 Lower menu bar The lower menu bar contains the shortcut keys or smart keys. The presentation will depend upon the configuration of the monitor. 1
1
Scroll left to show more shortcut keys
2
Scroll right to show more shortcut keys
2
19
EN
3.7
Product Labelling
Symbol Explanation This symbol signifies that this product, including its accessories and consumables is subject to the WEEE (Waste Electrical and Electronic Equipment) regulations and should be disposed of responsibly in accordance with local procedures. This symbol signifies that this product complies with the essential requirements of the Medical Device Directive (93/42/EEC) - Medical Device Regulation (EU/2017/745) Protection against vertically dripping when the device is tilted at an angle up to 15° from its normal position. Huntleigh Healthcare Ltd. 35 Portmanmoor Road, Cardiff, CF24 5HN, United Kingdom Manufactured By: T: +44 (0)29 20485885 [email protected] www.huntleigh-diagnostics.com Legal Manufacturer in association with the CE mark in Europe ArjoHuntleigh AB Hans Michelsensgatan 10 211 20 Malmö, Sweden IPX2
YYYY-MM
CF Applied parts, with defibrillation proof function
BF Applied parts, with defibrillation proof function
Warning
Attention, consult accompanying documents / Instructions for Use
~
Alternating current (AC)
On/Off
DI
Device Identifier
REF
Reference Number
SN
Serial Number (Date of manufacture is included in SN)
MD
Medical Device
50°C
-10°C
PVC
YYYY-MM
USB Port
Multi-function Interface
Network Interface
Ethernet Port
Keep Dry
Equipotential Earth
Fragile
Do not use hook
Temperature Limits
Cardboard packaging can be recycled.
Does not contain PVC
Humidity Limitations
Use By
LATEX
Not made with natural rubber latex.
Atmospheric Pressure Limits
Do Not Reuse
Max stack of x 4 identical boxes
This way up * As defined by IEC60601-1.
20