Arthrex
Angel cPRP System Operators Manual sw ver 1.21 Rev 0 June 2020
Operators Manual
108 Pages
Preview
Page 1
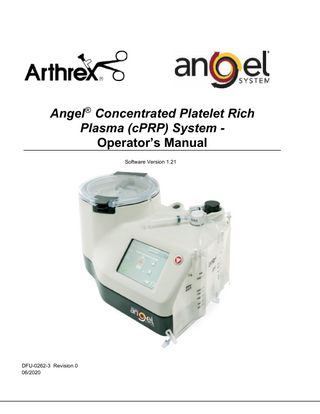
Angel® Concentrated Platelet Rich Plasma (cPRP) System Operator’s Manual Software Version 1.21
DFU-0262-3 Revision 0 06/2020
This page intentionally left blank
Overview
Table of Contents Before You Get Started Introduction ... vii Indications for Use ... vii Contraindications for Use ... vii Warnings ... vii Precautions ... x Symbols ... xiii Service Information ... xiv Return of Used Product ... xiv
Chapter 1: Overview Product Description ... 1-1 Description of the Angel® Concentrated Platelet Rich Plasma (cPRP) System ... 1-1 How the Angel System Works ... 1-1 Angel System Components ... 1-2 Shipping and Storage ... 1-3 Installation ... 1-3 Special tools, equipment and environmental requirements ... 1-4 Visual Inspection ... 1-4 Unpacking/Assembly ... 1-4 Operational Checks ... 1-6 Setting the Date and Time ... 1-6
Chapter 2: Installing the Angel cPRP Processing Set The Angel Processing Set or Disposable Set ... 2-1 Description ... 2-1 Warnings and Precautions ... 2-3 Setup and Blood/Bone Marrow Aspirate Preparation ... 2-3 Turning on the Angel System... 2-4
Chapter 3: Processing Before You Begin ... 3-1 Loading the Angel System ... 3-1 Collecting the Blood or Mixture of Blood and Bone Marrow ... 3-1 Running the Separation Process ... 3-2 Saving Case Data ... 3-6 Entering Optional Case Data Fields ... 3-7 Modifying Optional Data Field Values ... 3-8 Angel® cPRP System Operator’s Manual
i
Overview Selecting Past Cases ... 3-9 Saving a Tally Table to a USB Storage Device ... 3-10 Saving a Case Log ... 3-11 Touch Screen User Interface ... 3-12 Start Screen ... 3-12 Run Screen ... 3-14 End of Cycle Screen ... 3-16 End of Case Screen ... 3-17 Menu Screen ... 3-18 Information Screen ... 3-22 Past Cases Screen ... 3-23 Output Screen ... 3-24 Stop Button ... 3-26 Power Loss ... 3-27
Chapter 4: Programmability Option Creating Custom Protocols ... 4-1 Entering Values and Text ... 4-2 Creating a New Protocol ... 4-3 Editing the Parameters of a Protocol ... 4-4 Restoring the Parameters of a Protocol ... 4-4 Renaming a Protocol ... 4-5 Changing the Wakeup Protocol ... 4-5 Deleting a Protocol ... 4-5 The Protocols Tab ... 4-6 1. Protocol Buttons ... 4-7 2. Protocol Parameters and Buttons ... 4-7 3. Protocol Pull-Down Button ... 4-8 4. Close Button... 4-9 5-6. Up and Down Arrow Buttons ... 4-9 7. Information Button ... 4-9
Chapter 5: Troubleshooting Alarms and Notifications ... 5-1 Troubleshooting the Save Process ... 5-6 Troubleshooting the Software Update Process ... 5-6 Other Operational & Troubleshooting Tips ... 5-7
Chapter 6: Routine Care New Software ... 6-1 New Software Screen ... 6-4 Visual Inspection ... 6-5
ii
Angel® cPRP System Operator’s Manual
Overview Routine Care ... 6-5 Non-Routine Care ... 6-7 In the Event of a Serious Fluid (Blood) Spill ... 6-7 Dissassembling & Cleaning the Centrifuge Parts & Centrifuge Well ... 6-7 Preventive Maintenance Requirements ... 6-9 Fuse Replacement ... 6-10
Chapter 7: Technical Data Specifications ... 7-1 Performance Characteristics... 7-1 Physical Characteristics ... 7-1 Environmental Limitations ... 7-1
Chapter 8: Other Commercial Matters Limitation of Liability ... 8-1 Technical documentation ... 8-1 Technical safety standards ... 8-1 Identification of manufacturer... 8-3
Angel® cPRP System Operator’s Manual
iii
Overview
List of Figures Chapter 1: Overview Figure 1-1 Front-view of the Angel System ... 1-2 Figure 1-2 Rear-view of Angel System ... 1-2 Figure 1-3 Start Screen ... 1-3 Figure 1-4 Load Screen ... 1-6 Figure 1-5 Date and Time Settings ... 1-6
Chapter 2: Installing the Disposables Figure 2-1 Angel® Processing Set ... 2-2 Figure 2-2 Rear-view of Angel System ... 2-4 Figure 2-4 Centrifuge Stator Arm Aligned with Variable Volume Separation Chamber ... 2-9
Chapter 3: Processing Figure 3-1 Load Screen ... 3-1 Figure 3-2 Start Screen ... 3-2 Figure 3-3 Run Screen ... 3-3 Figure 3-4 End of Cycle Screen ... 3-3 Figure 3-5 End of Case Screen ... 3-4 Figure 3-6 “Tally” tab of the Menu Screen ... 3-7 Figure 3-7 Keyboard Screen (Text Entry) ... 3-8 Figure 3-8 Past Cases Screen ... 3-9 Figure 3-9 Output Screen ... 3-10 Figure 3-10 Start Screen ... 3-12 Figure 3-11 Run Screen ... 3-14 Figure 3-12 End of Cycle Screen ... 3-16 Figure 3-13 End of Case Screen ... 3-17 Figure 3-14 “Language” tab of the Menu Screen... 3-19 Figure 3-15 Tally Tab of the Menu Screen ... 3-20 Figure 3-16 “Settings” tab of the Menu Screen ... 3-21 Figure 3-17 Information Screen ... 3-22 Figure 3-18 Past Cases Screen ... 3-23 Figure 3-19 Output Screen ... 3-24 Figure 3-20 Empty Screen ... 3-26 Figure 3-21 Correct Valve Assembly Handle Position ... 3-27
iv
Angel® cPRP System Operator’s Manual
Overview
Chapter 4: Programmability Option Figure 4-1 The “Protocols” tab ... 4-2 Figure 4-2 The Keyboard Screen (Protocol Name) ... 4-2 Figure 4-3 “Protocols” tab of the Menu Screen ... 4-6
Chapter 6: Routine Care Figure 6-1 Unlock Code Screen... 6-2 Figure 6-2 Software Validated Screen ... 6-3 Figure 6-3 Install Screen ... 6-3 Figure 6-4 New Software Screen ... 6-4 Figure 6-7 Removing the Centrifuge Adaptor Plate ... 6-8 Figure 6-8 Removing the Spring Plate and Springs ... 6-9 Figure 6-9 Cleaning On and Around the Adaptor Base ... 6-9 Figure 6-10 Fuse Replacement: Pry Open Cover ... 6-10 Figure 6-11 Fuse Replacement: Slide Out Holders ... 6-10
This is not a warranty document. For all warranty information, including disclaimers, exclusions, terms, conditions and related provisions refer to the “Arthrex U.S. Product Warranty” section of the Arthrex, Inc. website, found at www.arthrex.com whose provisions are incorporated herein by reference.
Angel® cPRP System Operator’s Manual
v
Overview
This page intentionally left blank
vi
Angel® cPRP System Operator’s Manual
Overview
Before You Get Started Introduction The Angel® Concentrated Platelet Rich Plasma (cPRP) System (Angel System) is designed to separate autologous blood or a mixture of blood and bone marrow. The primary blood components that the Angel System separates and collects are red blood cells (RBC), platelet poor plasma (PPP) and platelet rich plasma (PRP). The Angel System utilizes a Variable Volume Separation Chamber that is capable of processing between 40 mL to 180 mL of anticoagulated whole blood or mixture of blood and bone marrow in a single cycle. A maximum of 180 mL can be processed in up to three (3) cycles for each disposable Angel® Concentrated Platelet Rich Plasma (cPRP) System Processing Set (Angel Processing Set or Disposable Set). This manual is intended for users of the Angel System. The procedures recommended in this Operator’s Manual have been developed and tested to provide safe, reliable and efficient operation of the Angel System. It is important that the operator thoroughly understand the information in this Operator’s Manual before attempting to use the Angel System.
Indications for Use The Arthrex Angel System is indicated to be used intraoperatively at the point of care for the safe and rapid preparation of autologous platelet poor plasma and platelet concentrate (platelet rich plasma) from a small sample of peripheral blood or a small sample of a mixture of peripheral blood and bone marrow. The platelet poor plasma and platelet rich plasma are mixed with autograft and/or allograft bone prior to application to a bony defect for improving handling characteristics. Disclaimer: Platelet Rich Plasma prepared from a mixture of whole blood and bone marrow may contain higher levels of plasma free hemoglobin than Platelet Rich Plasma prepared from whole blood.
Contraindications for Use The Angel System may be contraindicated in cases where there are active systemic infections or systemic heparinization.
Warnings 1. This device is intended to be used by a trained medical professional. A trained operator should be present at all times to operate and monitor the Angel System during processing. 2. Biohazard waste, such as needles and contaminated surgical equipment, should be safely disposed of in accordance with the institutions policy. Disposal of used equipment and/or used Angel Processing Sets should be performed in accordance with federal, state, and local regulations. These materials should be considered biohazardous. Universal precautions for blood-borne pathogens Angel® cPRP System Operator’s Manual
vii
Overview should be practiced (e.g., gloves, Personal Protective Equipment (PPE), etc.) when disposing of these items. 3. The use of operating or maintenance procedures other than those published by the manufacturer, or the use of accessory devices not recommended by the manufacturer may result in poor equipment performance. 4. The manufacturer will not be responsible for patient safety or equipment performance if the Angel System is operated in a manner other than specified in this manual. Medical individuals performing the operations described in this manual must be properly trained and qualified. 5. Any equipment modifications must be performed by qualified persons and be approved by the manufacturer in writing. 6. All electrical installations must comply with all applicable local electrical codes and the manufacturer’s specifications. 7. This equipment, when used with the specified data accessories, meets the following standards identified below. The user does not need to provide additional efforts regarding electromagnetic emissions or immunity: IEC 60601-1-2 o EN 55011, Class A standards o EN 61000 • Canadian Warning: This equipment is intended for use by healthcare professionals only. The Angel System may cause radio interference or may disrupt the operation of nearby equipment. It may be necessary to take mitigation measures, such as re-orienting or relocating the Angel System or shielding the location. o Use sterile technique when setting up the Angel Processing Set o Thoroughly clean and disinfect the donation site o Use sterile technique whenever handling autologous blood products 8. To avoid the risk of electrical shock, this equipment must only be connected to a supply mains with protective earth. Do not use alternate power plugs or adapters that disconnect the safety ground. 9. The operator should never touch the USB port on the Angel System, while at the same time making contact with the patient, as potential for electrical shock may result. •
10. Place the Angel System on a flat, stable surface. Never try to move the Angel System while the device is in operation. Failure to comply may result in damage to the Angel System and injury may result. 11. Do not use the Angel System in the presence of flammable agents as an explosion and/or fire may result. 12. Do not contact any moving parts of the centrifuge or pump while the Angel System is in operation. Injury may result. 13. Only Angel Processing Sets are approved for patient use with the Angel System. 14. Do not use the Angel Processing Set if the sterile packaging barrier has been broken. 15. Carefully examine the Angel Processing Set for damage prior to use. Should any evidence of damage to the Processing Set be evident, do not use the Angel Processing Set. 16. Carefully observe the Angel Processing Set for leaks during use. Leakage may
viii
Angel® cPRP System Operator’s Manual
Overview result in loss of sterility of the device or loss of blood product. 17. Use of this product for pediatric patients is at the discretion of a physician. Blood withdrawal from a pediatric patient should be performed in the presence and at the direction of a physician to prevent significant reduction of the circulating blood volume. 18. When collecting and processing autologous blood products, it is recommended that the following precautions be followed to insure that the autologous product is not contaminated: •
Use sterile technique when setting up the Angel Processing Set
•
Thoroughly clean and disinfect the donation site
• Use sterile technique whenever handling autologous blood products 19. The whole blood or the mixture of blood and bone marrow must be anticoagulated before it can be processed for separation. Inadequate anticoagulation may result in clotting, interfering with the processing of the blood products. Blood containing clots will not pass through the syringe-activated valve located on the Whole Blood Compartment of the Three-Compartment Reservoir Bag. 20. Failure to properly load the Centrifuge Plate prior to processing, may lead to exposure to blood and blood-borne pathogens. 21. If centrifugation is discontinued before the completion of a processing cycle, the Variable Volume Separation Chamber is pressurized and presents the risk for exposure to blood and blood-borne pathogens if the Variable Volume Separation Chamber is not properly removed. Please refer to “Stop Button” on page 3-26 for emptying a Variable Volume Separation Chamber containing blood. 22. If a power loss occurs, and there is blood or a mixture of blood and bone marrow in the Variable Volume Separation Chamber, follow the instructions under “Power Loss” on page 3-27. 23. Failure to properly secure the Luer Lock Syringe to the Valve Assembly may result in a leakage of fluids. 24. Do not connect the patient directly to the Three-Compartment Reservoir Bag. A direct connection to the patient could lead to vascular damage, shock, or an air embolism. 25. Do not place objects in or on the pump during pump rotation. Damage to the machine and Angel Processing Set may occur. 26. If the Angel System fails to operate as intended, do not use the separated blood products. 27. The platelet rich plasma is not intended for transfusion. 28. The Angel System is not intended to be used by the patient. As such a mains power switch is not available to the user. In case of an emergency, power from the unit can be removed by unplugging the unit from the electrical socket. 29. Only devices or cables meeting IEC 60950 and IEC 60601-1 should be connected to the USB Port. Failure to do so may result in operator shock. All cables used in conjunction with the device should be no longer than 1 m (3 ft.) in length. Operators connecting other devices to the USB port must ensure compliance to the system requirements of IEC 60601-1. 30. Operators connecting other devices to the USB Port must ensure compliance to the system requirements of IEC 60601-1. Connection of other devices could result in previously unidentified risk to the patient, operator, or third parties. It is responsibility of the operator to identify, analyze, evaluate, and control any Angel® cPRP System Operator’s Manual
ix
Overview previously unidentified risks. 31. The Potential Equalization Conductor (PEC) is a common ground point with the device that is connected directly to the power input ground. The PEC is used for the Angel System Electrical Safety Testing. The PEC is not to be used by the operator to connect additional medical devices to the system during installation, or use of the system. 32. Caution: Federal law restricts this device to sale by or on the order of a physician. 33. Serious incidents should be reported to Arthrex Inc., or an in-country representative, and to the health authority where the incident occurred.
Precautions 1. Due to the possibility of operator exposure to blood-borne pathogens (such as HIV, hepatitis viruses, bacteria, etc.), Universal Precautions for blood-borne pathogens should be practiced (e.g., gloves, Personal Protective Equipment (PPE), etc.). 2. The Angel Processing Set is intended for single patient use only. a. Each set can be used on the same patient for up to three sequential processing cycles. b. Once used, it should be disposed of properly. c.
Do not resterilize any part of this Processing Set.
d. The Processing Set should not be re-used for another patient. 3. Carefully read this Operator’s Manual for complete instructions. 4. Use a neutral-pH enzymatic detergent when cleaning the Platelet Sensor. Do not use bleach, abrasive materials or solvents, as they may cause damage to the sensor. 5. Do not place external light sources within 1 meter (3 ft.) of the unit when operating the Angel System. External light sources may interfere with the operation of the Platelet Sensor and may result in reduced processing efficiency. 6. Do not immerse the Pump Rotor in cleaning solution or autoclave, as this may result in damage. 7. Follow the installation instructions included in this manual, prior to first use. 8. To prevent risk of electrical shock, shut OFF the power and unplug the system from the electrical outlet before performing cleaning procedures or replacing the fuses. 9. Immediately report any of the following conditions to the Arthrex, Inc. Customer Service. Don’t use the Angel System until corrective action has been taken: • • • • •
Damaged or worn Power Cord Assembly, plug or receptacle Switches that are loose, or do not operate properly A system that has been subjected to physical damage A system that has electrically shocked anyone A system that appears to be overheating
10. It is the responsibility of the health care institution to adequately prepare and identify the product for return shipment. Do not return products that have been exposed to blood-borne infectious diseases. 11. When removing the Angel Processing Set from its packaging, check to ensure that
x
Angel® cPRP System Operator’s Manual
Overview the three (3) Threaded Luer Caps (see Figure 2-1, item 5) on each Compartment (Whole Blood, RBC and PPP) are securely tightened prior to installation into the Angel System. 12. Failure to properly load the Angel Processing Set, per the enclosed instructions may adversely affect the performance of the system. 13. Luer Lock Syringes should be used with the Angel Processing Set. 14. Pressing the Stop Button during separation may reduce processing efficiency. 15. The physician ordering the collection of PRP shall use discretion when any of the following conditions exist: • • • • • • •
sepsis preoperative hematocrit less than 30% preoperative platelet count less than 195,000 per µL hemodynamically unstable prolonged clotting times recent use of anti-platelet drugs inability to maintain stable oncotic pressure
16. Only attach the Power Cord to a power outlet that is properly grounded. 17. Replace the mains fuses only with fuses of the same type and rating. 18. There are no user-serviceable parts inside this device. To avoid the risk of electrical shock, do not remove the cover. Refer all servicing to qualified service personnel. 19. Federal law (USA) restricts this device to sale by or on the order of a physician. 20. If the case data are relevant for patient’s treatment, it will always be necessary to use other Hospital standard measuring instruments. 21. The user of the Angel System is responsible for the monitoring of the system performance, when using custom protocols. 22. The Angel System has been tested and verified to meet applicable Electromagnetic Compatibly (EMC) and Electrical Safety standards, when put into service according to this manual. (Refer to Chapter 7 - “Environmental Limitations”.) 23. The Angel System has the ability to save data using a USB connection. Note: Only devices or cables meeting IEC 60950 and IEC 60601-1 should be connected to the USB Port and/or Ethernet Port(if present). Failure to do so may result in operator shock. All cables used in conjunction with the device should be no longer than 1 m (3 ft.) in length. Operators connecting other devices to the USB ports must ensure compliance to the system requirements of IEC 60601-1. 24. The pins of the USB connector should not be touched, and connection to the port should not be made unless ESD (Electrostatic discharge) precautionary procedures are used. 25. Surgeons are advised to review the product-specific surgical technique prior to performing any surgery. Arthrex provides detailed surgical techniques in print, video, and electronic formats. The Arthrex website also provides detailed surgical technique information and demonstrations. Or, contact your Arthrex representative for an onsite demonstration Angel® cPRP System Operator’s Manual
xi
Overview
xii
Angel® cPRP System Operator’s Manual
Overview
Symbols The following symbols appear on the device labeling. All of the symbols used on the labeling along with the title, description and standard designation number may be found on our website at www.arthrex.com/symbolsglossary.
Catalog number
MODEL
2x F 5AL 250V
Model number
SN
Serial number
Follow instructions for use
This side up
Date of manufacture
Fragile
This symbol indicates that the device requires an alternating supply current.
Keep dry
This symbol identifies the point of connection of a potential equalization conductor. The location of this connection point is at the rear of the machine, below the access door.
Ship and store between these temperatures: -20 and 37.7 degrees C (-4 and 100 degrees F).
This symbol indicates the power OFF position on the main power switch.
Ship and store between 10% and 90% relative humidity.
This symbol indicates the power ON position on the main power switch.
Ship and store between 50 kPa and 106 kPa.
Manufacturer
Install and operate between 10 and 30 degrees C (50 and 86 degrees F).
Caution: Federal law (USA) restricts this device to sale by or on the order of a veterinarian.
Install and operate between 10% and 70% relative humidity.
This symbol specifies the type of fuse(s).
Install and operate between 70 kPa and 106 kPa.
Angel® cPRP System Operator’s Manual
xiii
Overview
Service Information The company accepts responsibility for the safety, reliability and performance of this equipment only if operational procedures, calibrations and repairs are performed by appropriately qualified persons; if all equipment modifications are authorized in writing by the company and performed by appropriately qualified persons; if the electrical installation of the relevant room complies with all applicable local electrical codes; and if the equipment is used in accordance with the published instructions for use. If you require technical assistance, please contact your Customer Service Representative. Arthrex, Inc. 1370 Creekside Blvd Naples, FL 34108 USA Telephone: + 1 800-391-8599 [email protected] www.arthrex.com
Return of Used Product If for any reason this product must be returned to Arthrex, Inc., a Returned Materials Authorization (RMA) number is required from Arthrex, Inc. prior to shipping it. If the product has been in contact with blood or body fluids, it must be thoroughly cleaned and disinfected before packing. It should be shipped in either the original carton, or an equivalent carton, to prevent damage during shipment; and it should be properly labeled with the RMA number and an explanation of the biohazardous nature of the contents. Instructions for cleaning and materials, including appropriate shipping containers, proper labeling and an RMA number may be obtained from the Arthrex, Inc. Customer Service (+1 800-391-8599) or [email protected]. PRECAUTION It is the responsibility of the health care institution to adequately prepare and identify the product for its return. Do not return products that have been exposed to blood-borne infectious diseases. The shipping address for returned goods is: Arthrex, Inc. 14550 Plantation Road Fort Myers, FL 33912 Telephone: + 1 800-391-8599 [email protected] www.arthrex.com
xiv
Angel® cPRP System Operator’s Manual
Overview
This page intentionally left blank
Angel® cPRP System Operator’s Manual
xv
Overview
Chapter 1: Overview Product Description The Angel® Concentrated Platelet Rich Plasma (cPRP) System (Angel System) consists of a blood processing system and disposable products used for separation of whole blood or a mixture of blood and bone marrow into red cells, platelet poor plasma, and platelet rich plasma. The disposable Angel® Concentrated Platelet Rich Plasma (cPRP) System Processing Set is designed for single-patient use. The Variable Volume Separation Chamber allows the clinician to process from 40 mL to 180 mL of autologous whole blood or a mixture of blood and bone marrow in a single cycle. A maximum of 180 mL can be processed in up to three (3) cycles for each disposable Angel Processing Set.
Description of the Angel® Concentrated Platelet Rich Plasma (cPRP) System How the Angel System Works The Angel System processes a determined volume of anticoagulated whole blood or a mixture of blood and bone marrow from a patient and separates the blood/bone marrow into its primary components: red blood cells (RBC), platelet poor plasma (PPP), and platelet rich plasma (PRP). The basic steps are: Blood Collection: The whole blood or a mixture of blood and bone marrow is drawn from a patient and mixed with a citrate anticoagulant. The collected whole blood/bone marrow is mixed in a 7:1 ratio (7 parts whole blood to 1 part citrate anticoagulant (ACD-A)). Please refer to the Instructions for Use for the Angel® Concentrated Platelet Rich Plasma (cPRP) Processing Set for details regarding Blood Collection for further details. Processing: The Angel Processing Set utilizes a Variable Volume Separation Chamber (40 mL to 180 mL) which allows the clinician to determine the amount of preoperative blood/bone marrow volume to be processed. Each Angel Processing Set can be used for up to three processing cycles. Once the anticoagulated whole blood or mixture of blood and bone marrow has been dispensed into the Whole Blood Compartment of the reservoir bag, the clinician selects the desired volume of autologous whole blood/bone marrow to process and presses the “Start” Button on the Touch Screen Display. The Angel System will fill the Variable Volume Separation Chamber with the pre-determined volume of anticoagulated whole blood or a mixture of blood and bone marrow, separate the whole blood/bone marrow through centrifugation, and collect the primary blood components (RBC, PPP, and PRP) in their respective collection compartments. Administration: Reinfusion of blood components is under the control and supervision of the physician in charge. Follow your institution’s blood administration protocol for appropriate handling and labeling of blood components.
Angel® cPRP System Operator’s Manual
1-1
Overview
Angel System Components
1. 2. 3. 4.
Centrifuge Well Lid Latch Release Handle Lid Pump Rotor
5. Valve Assembly Driver 6. Stop Button 7. Touch Screen Display
Figure 1-1 Front-view of the Angel System
1. Power Cord 2. Power Switch
3. USB Port
Figure 1-2 Rear-view of Angel System
1-2
Angel® cPRP System Operator’s Manual
Overview
Touch Screen Display User Interface The color touch screen provides both the controls and the necessary information for operating the Angel System. Below is the start screen.
Figure 1-3 Start Screen
Shipping and Storage 1. Ship and store the carton in an upright position. 2. The contents are fragile. Do not drop, jar or shake the carton. 3. Keep dry. Ship and store between 10% and 90% relative humidity. 4. Store between -20 and 37.7 degrees Celsius (-4 to 100 degrees Fahrenheit). 5. Store between 50 kPa and 106 kPa atmospheric pressure.
Installation This section contains installation instructions for the Angel System. The Angel System has been designed to be a “plug and play” device and it requires very little preparation to get started. Before proceeding, please note the following: 1. Read the installation procedure in its entirety. 2. Become familiar with any precautionary instructions in this procedure. Note: If problems with the installation occur, contact Arthrex, Inc. Customer Service at +1 800-391-8599 or [email protected]
Angel® cPRP System Operator’s Manual
1-3