Arthrex
Angel cPRP System Processing Set Instructions for Use Rev 0 May 2021
Instructions for Use
8 Pages
Preview
Page 1
Angel® Concentrated Platelet-Rich Plasma (cPRP) Processing Set GB -
ENGLISH
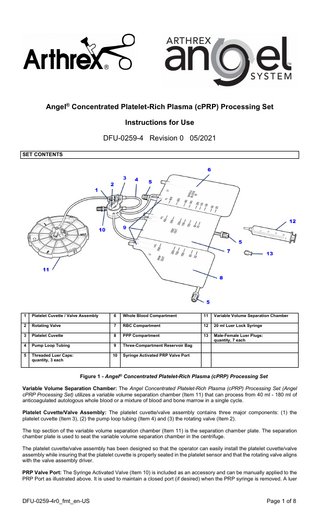
SET CONTENTS
Instructions for Use DFU-0259-4 Revision 0 05/2021 GB – ENGLISH - INSTRUCTIONS FOR USE
1
Platelet Cuvette / Valve Assembly
6
Whole Blood Compartment
11
Variable Volume Separation Chamber
2
Rotating Valve
7
RBC Compartment
12
20 ml Luer Lock Syringe
3
Platelet Cuvette
8
PPP Compartment
13
4
Pump Loop Tubing
9
Three-Compartment Reservoir Bag
Male-Female Luer Plugs: quantity, 7 each
5
Threaded Luer Caps: quantity, 3 each
10
Syringe Activated PRP Valve Port
Figure 1 - Angel® Concentrated Platelet-Rich Plasma (cPRP) Processing Set Variable Volume Separation Chamber: The Angel Concentrated Platelet-Rich Plasma (cPRP) Processing Set (Angel cPRP Processing Set) utilizes a variable volume separation chamber (Item 11) that can process from 40 ml - 180 ml of anticoagulated autologous whole blood or a mixture of blood and bone marrow in a single cycle. Platelet Cuvette/Valve Assembly: The platelet cuvette/valve assembly contains three major components: (1) the platelet cuvette (Item 3), (2) the pump loop tubing (Item 4) and (3) the rotating valve (Item 2). The top section of the variable volume separation chamber (Item 11) is the separation chamber plate. The separation chamber plate is used to seat the variable volume separation chamber in the centrifuge. The platelet cuvette/valve assembly has been designed so that the operator can easily install the platelet cuvette/valve assembly while insuring that the platelet cuvette is properly seated in the platelet sensor and that the rotating valve aligns with the valve assembly driver. PRP Valve Port: The Syringe Activated Valve (Item 10) is included as an accessory and can be manually applied to the PRP Port as illustrated above. It is used to maintain a closed port (if desired) when the PRP syringe is removed. A luer
DFU-0259-4r0_fmt_en-US
Page 1 of 8
lock syringe is attached to the Syringe Activated PRP Valve Port to collect PRP. At the end of a processing cycle, the PRP valve port can also be used to collect PPP by drawing back on the PRP syringe plunger. Three-Compartment Reservoir Bag: The three-compartment reservoir bag is used to collect anticoagulated whole blood, the mixture of blood and bone marrow, and the separated blood components. The whole blood compartment is used as a reservoir for collected anticoagulated whole blood/ bone marrow from a patient. The clinician may use syringes or whole blood bags to collect anticoagulated whole blood or a mixture of blood and bone marrow from a patient. The RBC Compartment is used to collect the concentrated red cells at the end of the processing cycle. The PPP Compartment is used to collect platelet poor plasma; the PPP is the first blood component collected after separation has been completed. Syringe activated valves are used to access the PPP and whole blood compartments of the three-compartment reservoir bag. Other items in the Angel cPRP Processing Set include: 20 ml Luer Lock Syringe: The 20 ml luer lock syringe is used for the collection of platelet rich plasma. However, the syringe activated PRP valve will accommodate most luer fitting syringes. Male/Female Luer Plugs: The male/female luer plugs can be used during and at the end of the procedure to seal open luer lock connections. Labels: Appropriate labels supplied with kit, to label collected whole blood and separated components. DESCRIPTION The Angel cPRP Processing Set consists of a pre-connected variable volume separation chamber, a tubing set with a platelet sensor/valve assembly and a three-compartment reservoir bag for the collection of blood products (whole blood, red blood cells and platelet poor plasma respectively). The Angel cPRP Processing Set also contains a 20ml luer lock syringe for the collection of platelet rich plasma (PRP), male-female luer plugs and labels for collected blood components. Contents of this set have been sterilized by ethylene oxide gas and have non-pyrogenic fluid pathway. INDICATIONS FOR USE The Angel® System is indicated to be used intraoperatively at the point of care for the safe and rapid preparation of autologous platelet poor plasma and platelet concentrate (platelet rich plasma) from a small sample of peripheral blood or a small sample of a mixture of peripheral blood and bone marrow. The platelet poor plasma and platelet rich plasma are mixed with autograft and/or allograft bone prior to application to a bony defect for improving handling characteristics. Disclaimer: Platelet rich plasma prepared from a mixture of whole blood and bone marrow may contain higher levels of plasma-free hemoglobin than platelet rich plasma prepared from whole blood. CONTRAINDICATIONS The Angel cPRP Processing Set may be contraindicated in cases where there is active systemic infection or systemic heparinization. WARNINGS 1. This device is intended to be used by a trained medical professional. 2. Only Angel cPRP Processing Sets are approved for use with the Angel® Concentrated Platelet-Rich Plasma (cPRP) System. 3. Do not use the Angel cPRP Processing Set if the sterile packaging barrier has been broken. 4. Carefully examine the Angel cPRP Processing Set for damage, prior to use. Do not use if the Processing Set is damaged. 5. Carefully observe the Angel cPRP Processing Set for leaks during use. Leakage may result in loss of sterility of the device and/or loss of blood product. 6. When collecting and processing autologous blood products or a mixture of blood and bone marrow, it is recommended that the following precautions be followed to insure that the autologous product is not contaminated. • Use sterile technique when setting up the Angel cPRP Processing Set. • Thoroughly clean and disinfect the donation site. • Use sterile technique whenever handling autologous blood products. 7. The whole blood or the mixture of blood and bone marrow must be anticoagulated before it can be processed for separation. Inadequate anticoagulation may result in clotting, interfering with the processing of the blood products. Blood/ bone marrow containing clots will not pass through the syringe-activated valve located on the Whole Blood compartment of the three-compartment reservoir bag.
DFU-0259-4r0_fmt_en-US
Page 2 of 8
8. If centrifugation is discontinued before the completion of a processing cycle, the variable volume separation chamber is pressurized and presents the risk for exposure to blood and blood borne pathogens if the variable volume separation chamber is not properly emptied and removed. Please refer to the Angel Concentrated Platelet-Rich Plasma (cPRP) System Operator’s Manual (Chapter 3, Power Loss) for unloading a variable volume separation chamber containing blood. 9. Failure to properly secure the luer lock syringe to the syringe-activated valve on the valve assembly may result in a leakage of fluids. 10. Do not directly connect the patient to the three-compartment reservoir bag. Direct connection to the patient could lead to vascular damage, shock or air embolism. 11. Biohazard waste, such as needles and contaminated surgical equipment, should be safely disposed of in accordance with the institutions policy. Disposal of Angel cPRP Processing Sets should be in accordance with federal, state, and local regulations. These materials should be considered biohazardous. Universal precautions for blood borne pathogens should be practiced when disposing of these items. 12. Place the Angel Concentrated Platelet-Rich Plasma (cPRP) System on a flat, stable surface. Never try to move the Angel Concentrated Platelet-Rich Plasma (cPRP) System while the device is in operation. 13. The platelet rich plasma is not intended for transfusion. 14. Caution: Federal law restricts this device to sale by or on the order of a physician. 15. Serious incidents should be reported to Arthrex Inc., or an in-country representative, and to the health authority where the incident occurred. PRECAUTIONS 1. Carefully read these Instructions for Use before using this product. Refer to the Angel Concentrated Platelet-Rich Plasma (cPRP) System Operator’s Manual for complete instructions. 2. Due to the possibility of operator exposure to blood borne pathogens (such as HIV, hepatitis viruses, bacteria, etc.), Universal Precautions for blood borne pathogens should be practiced. 3. When removing the Angel cPRP Processing Set from its packaging, check to ensure that the three (3) Threaded Luer Caps (see Figure 1, item 5) on each Compartment (Whole Blood, RBC and PPP) are securely tightened priorto installation into the Angel System. 4. The Angel cPRP Processing Set is intended for a single use. Do not resterilize any part of this Processing Set. 5. Failure to properly load the Angel cPRP Processing Set per the enclosed instructions may affect the performance of the system. 6. Luer lock syringes should be used with the Angel cPRP Processing Set. 7. The safety and effectiveness of this device for in-vivo indications for use has not been established. 8. Surgeons are advised to review the product-specific surgical technique prior to performing any surgery. Arthrex provides detailed surgical techniques in print, video, and electronic formats. The Arthrex website also provides detailed surgical technique information and demonstrations. Or, contact your Arthrex representative for an onsite demonstration INSTRUCTIONS FOR USE Turning on the Angel cPRP System Turn on the Angel Concentrated Platelet-Rich Plasma (cPRP) System by pressing the power switch on the back of the machine (see Figure 2). The message “Self test in progress. Please stand by.” will be displayed on the Angel cPRP System’s touch screen display. The machine will then orientate the valve assembly driver to the loading position. Initial Setup With the Angel cPRP System turned on, perform the following steps: 1. Open the centrifuge lid cover and lift the centrifuge stator arm to lock the centrifuge adapter within the centrifuge well. 2. Remove the Angel cPRP Processing Set from the tray. 3. Lay the Angel cPRP Processing Set on the top of the machine. 4. Check to ensure that the three (3) Threaded Luer Caps (Figure 1, item 5) on each Compartment (Whole Blood, RBC and PPP) are securely tightened prior to proceeding to the next step.
DFU-0259-4r0_fmt_en-US
Page 3 of 8
Figure 2 - Rear-view of Angel Concentrated Platelet-Rich Plasma (cPRP) System 5. Insert the variable volume separation chamber into the centrifuge adapter by aligning the notches in the separation chamber plate down near the location of the position indicator and turn clockwise until the position indicator snaps into place (see Figure 3). 6. Rotate the centrifuge to a position so that the interlock mechanism shown in Figure 3 does not interfere with the stator arm. If the interlock mechanism interferes with the stator arm, the separation chamber plate will not load properly. Note: Loading the variable volume separate chamber should always be the first step in the setup process. Loading the variable volume separation chamber and pressing down on the separation chamber plate will remove the excess air volume from the chamber. If the excess air is not removed, the separation chamber plate will not load properly.
1
Separation Chamber Plate
2
Separation Chamber Plate Interlock Mechanism
3
Position Indicators
Figure 3 - Mounting the Separation Chamber 7.
Place the tube leading from the variable volume separation chamber through the slot on the rim of the centrifuge well.
8.
Lower the centrifuge stator arm and align it with the raised tab on the top of the rotating seal of the variable volume separation chamber (see Figure 4).
DFU-0259-4r0_fmt_en-US
Page 4 of 8
Figure 4 - Centrifuge stator arm aligned with the variable volume separation chamber
9. Close the centrifuge lid. After closing the centrifuge lid, make sure that the tubing remains in the slot on the rim of the centrifuge and is not occluded / pinched by the centrifuge lid. 10. Place the pump loop tubing over the pump rotor. The pump loop will automatically load when the processing cycle is initiated. Seat the platelet cuvette/valve assembly by aligning the platelet cuvette and the valve assembly with the platelet sensor body and the valve assembly driver. Press down firmly on the back side of the platelet cuvette/valve assembly, at position A near the pump loop, until the assembly is snapped in place (see label A, Figure 5). Note: It is essential that the platelet cuvette/valve assembly seats fully on the machine, to obtain proper sensing of the blood components.
1
Platelet Cuvette
3
Valve Assembly Driver
2
Valve Assembly
4
Syringe Activated PRP Valve
Figure 5 - Valve Assembly
11. Hang the three-compartment reservoir bag on the two support pins located on the side of the Angel Concentrated Platelet-Rich Plasma (cPRP) System. 12. Remove the breather cap from the PRP valve port located on the valve assembly. Attach the Syringe Activated PRP Valve to the PRP valve port. Remove the breather cap from the Syringe Activated PRP Valve. Prior to installation onto the PRP Valve Port, cycle (break loose) the syringe plunger then attach the 20 ml luer lock syringe (or alternate syringe, if desired), to the Syringe Activated PRP Valve..
DFU-0259-4r0_fmt_en-US
Page 5 of 8
Note: The luer on the Syringe Activated PRP Valve will accommodate most luer lock syringes. 13. After set-up, inspect the tube set to make sure there are no kinks or occlusions. Blood Collection The Angel Concentrated Platelet-Rich Plasma (cPRP) System utilizes a variable volume separation chamber that is capable of processing between 40 ml and 180 ml of anticoagulated whole blood or a mixture of blood and bone marrow (bone marrow aspirate [BMA]) in a single cycle. The Angel Concentrated Platelet-Rich Plasma (cPRP) System can accommodate anticoagulated whole blood or anticoagulated bone marrow aspirate that has been collected in syringes or blood collection bags. In either situation, the whole blood or BMA should be collected with citrate anticoagulant (ACD-A) in a 7:1 ratio (seven parts whole blood to one part citrate anticoagulant). The following table defines the appropriate mixture of whole blood or BMA and citrate anticoagulant (ACD-A): Whole Blood or Bone Marrow Aspirate (BMA) vs. Citrate Anticoagulant Mixture (7:1 ratio: seven parts blood or BMA to one part citrate anticoagulant) Total Volume of Anticoagulated Whole Blood/ Bone Marrow Aspirate (mL)
Volume of ACD-A (mL)
Total Volume of Whole Blood/ Bone Marrow Aspirate Drawn (mL)
40i 50 60 70 80 90 100 110 120 130 140 150 160 170 180
5 6 8 9 10 12 13 14 15 16 18 19 20 21 23
35 44 52 61 70 78 87 96 105 114 122 131 140 149 157
i40 ml anticoagulated whole blood volumes or bone marrow aspirate require a patient hematocrit of 30% or
greater. The recommended minimum patient hematocrit for anticoagulated whole blood or bone marrow aspirate volumes of 50 ml or greater is 28%.
During and after collection, gently mix the whole blood or bone marrow aspirate with the citrate anticoagulant for a thorough distribution of the anticoagulant. Failure to properly mix the collected blood or bone marrow aspirate with anticoagulant may cause blood clot formation. Blood clot formation may interfere with the loading of blood/ bone marrow aspirate into the Whole Blood compartment of the three-compartment reservoir bag and/or may interfere with the processing of the blood or bone marrow aspirate. If a syringe is used to collect blood or bone marrow aspirate, attach the syringe to the syringe-activated valve located on the Whole Blood compartment of the three-compartment reservoir bag and inject the blood or bone marrow aspirate. If using a whole blood collection bag to collect blood or bone marrow aspirate, ensure that the blood-citrate/ BMA-citrate ratio is correct by weighing the bag as the blood/ bone marrow aspirate is collected according to AABB standard methods.1 Place the citrated (ACD-A) blood bank bag on a standard metric scale and zero it prior to beginning to withdraw the blood or bone marrow aspirate. Refer to the instructions for the specific bag that you are using and allow the blood/ bone marrow aspirate to gravity drain into the bag until its weight equals the volume of the bag (1 ml of blood weighs approximately 1.053 grams). If bone marrow aspirate is to be processed using the Angel Concentrated Platelet-Rich Plasma (cPRP) System, samples of a patient's bone marrow aspirate should be obtained using the techniques and procedures as practiced at each individual hospital or health-care setting. Other than the capacity of the Whole Blood Compartment, there are no volume limitations of anti-coagulated whole blood and anticoagulated bone marrow aspirate that can be transferred into the whole blood compartment of the three-compartment reservoir bag using the syringe activated valve. After the blood or bone marrow aspirate has drained into the Whole Blood compartment of the three-compartment reservoir bag, remove the collection syringe or collection bag then recap the syringe-activated valve. Refer to the Angel Concentrated Platelet-Rich Plasma (cPRP) System Operator’s Manual for processing instructions. ______________________________________________________________________
1 Method 9.3 Phlebotomy and Collection. Technical Manual. American Association of Blood Banks. AABB Press. Bethesda, MD. 1996.
DFU-0259-4r0_fmt_en-US
Page 6 of 8
RETURN OF USED PRODUCT For Customers In Non CE Accepting Countries If for any reason this product must be returned to the manufacturer or to Arthrex, Inc., a returned goods authorization (RGA) number is required prior to shipping. If the product has been in contact with blood or body fluids, it must be thoroughly cleaned and disinfected before packing. It should be shipped in either the original carton, or an equivalent carton, to prevent damage during shipment; and it should be properly labeled with an RGA number and an indication of the biohazardous nature of the contents of the shipment. Instructions for cleaning and materials, including appropriate shipping containers, proper labeling, and an RGA number may be obtained from Arthrex Customer Service: Phone: +1 (800) 934-4404 Email: [email protected]
It is the responsibility of the health care institution to adequately prepare and identify the product for return shipment. Do not return products that have been exposed to blood borne infectious diseases. For Customers In CE Accepting Countries If for any reason this product must be returned, please contact your sales representative for specific instructions. If the product has been in contact with blood or body fluids, it must be thoroughly cleaned and disinfected before packing. It should be shipped in either the original carton, or an equivalent carton, to prevent damage during shipment.
It is the responsibility of the health care institution to adequately prepare and identify the product for return shipment. Do not return products that have been exposed to blood borne infectious diseases. TECHNICAL SUPPORT Call the Arthrex Technical Support Hotline at (800) 391-8599, Monday through Friday from 9:00 AM to 5:00 PM EST or [email protected]; at +49 89 909005 8800 or [email protected] from 8:00 AM to 5:00 PM CET. DEFINITION OF SYMBOLS (as used in product labeling) FOR SINGLE USE ONLY (DO NOT REUSE) BATCH CODE (NUMBER) (REFERENCE FOR PRODUCT TRACEABILITY)
SHIP AND STORE BETWEEN -20°C AND 60°C.
QTY QUANTITY
USE BY - YEAR/MONTH/DAY
CONSULT INSTRUCTIONS FOR USE
DATE OF MANUFACTURE
THIS WAY UP
STERILE - ETHYLENE OXIDE
CAUTION
NON PYROGENIC
FRAGILE; HANDLE WITH CARE
CONTAINS PHTHALATE
KEEP AWAY FROM HEAT
DOES NOT CONTAIN NATURAL RUBBER LATEX
KEEP DRY
WARNING: DO NOT RESTERILIZE.
MANUFACTURER
CONTENTS STERILE ONLY IF PACKAGE IS NOT OPENED, DAMAGED OR BROKEN
FEDERAL LAW (USA) RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN
CATALOG NUMBER All of the symbols used on the labeling along with the title, description and standard designation number may be found on our website at www.arthrex.com/symbolsglossary. This is not a warranty document. For all warranty information, including disclaimers, exclusions, terms, conditions and related provisions, refer to the “Arthrex U.S. Product Warranty” section of the Arthrex, Inc. website, found at www.arthrex.com whose provisions are incorporated herein by reference.
DFU-0259-4r0_fmt_en-US
Page 7 of 8