BBraun
activ C-insertion instrument
84 Pages
Preview
Page 1
Aesculap®
Aesculap Spine
en USA
de fr es it pt nl da sv fi lv lt ru cs pl sk hu sl hr ro bg tr el
Instructions for use/Technical description activ C-insertion instrument Note for U.S. users This Instructions for Use is NOT intended for United States users. Please discard. The Instructions for Use for United States users can be obtained by visiting our website at www.aesculapImplantsystems.com. If you wish to obtain a paper copy of the Instructions for Use, you may request one by contacting your local Aesculap representative or Aesculap's customer service at 1-866-229-3002. A paper copy will be provided to you upon request at no additional cost. Gebrauchsanweisung/Technische Beschreibung activ C-Einsetzinstrument Mode d’emploi/Description technique Instrument d'insertion activ C Instrucciones de manejo/Descripción técnica Instrumental de inserción activ C Istruzioni per l’uso/Descrizione tecnica Strumento inseritore activ C Instruções de utilização/Descrição técnica Instrumento introdutor activ C Gebruiksaanwijzing/Technische beschrijving actief C-inbrenginstrument Brugsanvisning/Teknisk beskrivelse activ C-indføringsinstrument Bruksanvisning/Teknisk beskrivning activ C-insättningsinstrument Käyttöohje/Tekninen kuvaus Sisäänvienti-instrumentti activ C Lietošanas instrukcijas/tehniskais apraksts activ C ievietošanas instruments Naudojimo instrukcija/techninis aprašas activ C įdėjimo instrumentas Инструкция по примению/Техническое описание Инструмент для установки activ C Návod k použití/Technický popis Zaváděcí nástroj activ C Instrukcja użytkowania/Opis techniczny Narzędzie do wprowadzania activ C Návod na použitie/Technický opis activ C - vkladací nástroj Használati útmutató/Műszaki leírás activ C beültető műszer Navodila za uporabo/Tehnični opis Instrument za vstavljanje activ C Upute za uporabu/Tehnički opis activ C instrument za umetanje Manual de utilizare/Descriere tehnică Instrument de inserție activ C Упътване за употреба/Техническо описание Инструмент за вкарване activ C Kullanım Kılavuzu/Teknik açiklama activ C- insersiyon aleti Οδηγίες χρήσης/Τεχνική περιγραφή Εργαλείο εισαγωγής activ C
Aesculap AG | Am Aesculap-Platz | 78532 Tuttlingen | Germany Phone +49 (0) 7461 95-0 | Fax +49 (0) 7461 95-26 00 | www.aesculap.com Aesculap® – a B. Braun brand TA011997
0482
2021-05
V6
Change No. AE0060911
3
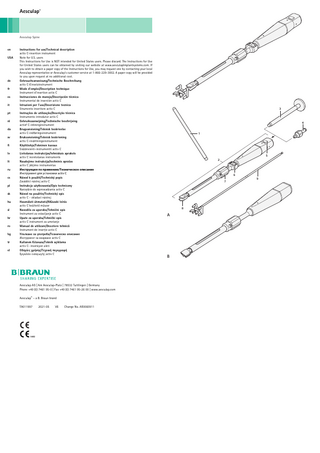
4 1
5 2
6 7
8
A
B
9
en
2.3.2 ®
Aesculap activ C-insertion instrument Legend A Insertion instrument and accessories 1 Key (for insertion instrument) 2 Insertion instrument 3 Repositioning instrument 4 Irrigation connector 5 Button 6 Adjusting wheel 7 Clamping sleeve 8 Connector piece 9 Spacer B Revision instrument
1.
About this document
Note General risk factors associated with surgical procedures are not described in these instructions for use.
1.1
2.4
Application
WARNING Risk of injury and/or malfunction! ► Prior to each use, inspect the product for loose, bent, broken, cracked, worn, or fractured components. ► Always carry out a function test prior to each use of the product. ► Follow OP-Manual no. O31302 and instructions for use “activ C disk prosthesis”, see TA011995. ► Mounting the insertion instrument, see Assembly.
2.4.1
Coupling the implant
WARNING Damage to the implant due to improper handling! ► Follow the instructions for use of activ C disk prosthesis TA011995. ► Mount the implant on the insertion instrument immediately after removing it from the implant packaging. ► Handle the friction surfaces of the prosthesis plates and of the polyethylene inlay with care. ► Open the implant packaging.
Note Do not remove the implant from the packaging! ► Mount insertion instrument 2 on the disc prosthesis with the latter still in its packaging, observing the
Scope
These instructions for use apply for activ C-insertion instruments. Note The applicable CE mark for the product can be seen on the label or packaging of the product. ► For article-specific instructions for use as well as information on material compatibility and lifetime see B. Braun
eIFU at eifu.bbraun.com
1.2
Sterility
The product is delivered in an unsterile condition. ► Clean the new product after removing its transport packaging and prior to its initial sterilization.
Safety messages
Safety messages make clear the dangers to patient, user and/or product that could arise during the use of the product. Safety messages are labeled as follows:
"CRANIAL" and "CAUDAL" markings. The superior prosthesis plate (the one with the spikes) must abut the part of insertion instrument 2 marked "CRANIAL", while the inferior prosthesis plate (with the fin) has to abut the part of insertion instrument 2 marked "CAUDAL". ► Turn clamping sleeve 7 clockwise to fixate the intervertebral disc prosthesis on insertion instrument 2. ► Remove the disc prosthesis from its packaging, holding it with the insertion instrument 2.
2.4.2
Inserting the implant
WARNING Damage to vertebral body end plates when the intervertebral disk prosthesis is tapped in! ► Apply proper care when tapping in the intervertebral disk prosthesis.
DANGER Indicates a possible threat of danger. If not avoided, death or serious injury may result.
► Carefully, under X-ray control, insert the disk prosthesis into the intervertebral disk space. The superior prosthesis
WARNING Indicates a possible threat of danger. If not avoided, minor or moderate injury may result.
► If necessary impel the implant with light hammer taps on the proximal end of insertion instrument 2.
CAUTION Indicates a possible threat of material damage. If not avoided, the product may be damaged.
2.
Clinical use
2.1
Available sizes
plate (plate with the spikes) must be oriented cranially, the inferior prosthesis plate (the plate with the fin) caudally.
WARNING Compression of the spinal canal and of other posterior elements caused by the intervertebral disk prosthesis inserted too deeply! ► Prior to inserting the intervertebral disk prosthesis, adjust the depth stop to minimum insertion depth. ► Introduce the disk prosthesis into the intervertebral space under X-ray control. ► If necessary, correct the depth stop position using the adjusting wheel 6 until the intended position is reached. ► Should repositioning become necessary, screw repositioning instrument 3 onto the proximal end of insertion
instrument 2 and carefully, with a slotted hammer, move the implant to its intended position. Art. no.
Designation
2.4.3 FW857R
Insertion instrument with irrigation connector H6
FW863R
Spacer for implant height 5 mm
FW864R
Spacer for implant height 6 mm
Decoupling the implant
Note To avoid damage to the implant, do not couple an insertion instrument that already has been decoupled once. ► Loosen clamping sleeve 7 on insertion instrument 2 by turning it counterclockwise, and remove insertion
instrument 2. ► If clamping sleeve 7 is difficult to loosen, insert key 1 in one of the lateral drill holes of clamping sleeve 7 and
FW866R
loosen clamping sleeve 7 in this way.
Insertion instrument with irrigation connector H5
► On the intraoperative X-ray image, in AP and lateral view, check the size, height and position of the intervertebral
FW867R
Repositioning instrument
FW868R
Revision instrument
3.
Validated reprocessing procedure
FW945R
Key for insertion instrument
3.1
General safety instructions
2.2
Areas of use and limitations of use
2.2.1
Intended use
The insertion instrument is used for picking up the activ C intervertebral disc prosthesis from its sterile packaging, inserting it in the prepared intervertebral space, and carrying out position corrections of the prosthesis.
2.2.2
Indications
Note The manufacturer is not responsible for any use of the product against the specified indications and/or the described applications. For indications, see Intended use.
2.2.3
Absolute contraindications
No known absolute contraindications.
2.2.4
Relative contraindications
disk implant.
Note Adhere to national statutory regulations, national and international standards and directives, and local, clinical hygiene instructions for sterile processing. Note For patients with Creutzfeldt-Jakob disease (CJD), suspected CJD or possible variants of CJD, observe the relevant national regulations concerning the reprocessing of products. Note Mechanical reprocessing should be favored over manual cleaning as it gives better and more reliable results. Note Successful processing of this medical device can only be ensured if the processing method is first validated. The operator/sterile processing technician is responsible for this. Note If there is no final sterilization, then a virucidal disinfectant must be used.
The following conditions, individual or combined, can lead to delayed healing or compromise the success of the operation: ■ Medical or surgical conditions (e.g. comorbidities) which could hinder the success of the operation. In the presence of relative contraindications, the user decides individually regarding the use of the product.
Note For up-to-date information about reprocessing and material compatibility, see B. Braun eIFU at eifu.bbraun.com The validated steam sterilization procedure was carried out in the Aesculap sterile container system.
2.3
Safety information
2.3.1
Clinical user
Dried or affixed surgical residues can make cleaning more difficult or ineffective and lead to corrosion. Therefore the time interval between application and processing should not exceed 6 h; also, neither fixating pre-cleaning temperatures >45 °C nor fixating disinfecting agents (active ingredient: aldehydes/alcohols) should be used. Excessive measures of neutralizing agents or basic cleaners may result in a chemical attack and/or to fading and the laser marking becoming unreadable visually or by machine for stainless steel. Residues containing chlorine or chlorides e.g. in surgical residues, medicines, saline solutions and in the service water used for cleaning, disinfection and sterilization will cause corrosion damage (pitting, stress corrosion) and result in the destruction of stainless steel products. These must be removed by rinsing thoroughly with demineralized water and then drying. Additional drying, if necessary. Only process chemicals that have been tested and approved (e.g. VAH or FDA approval or CE mark) and which are compatible with the product’s materials according to the chemical manufacturers’ recommendations may be used for processing the product. All the chemical manufacturer's application specifications must be strictly observed. Failure to do so can result in the following problems: ■ Optical changes of materials, e.g. fading or discoloration of titanium or aluminum. For aluminum, the application/process solution only needs to be of pH >8 to cause visible surface changes. ■ Material damage such as corrosion, cracks, fracturing, premature aging or swelling. ► Do not use metal cleaning brushes or other abrasives that would damage the product surfaces and could cause corrosion. ► Further detailed advice on hygienically safe and material-/value-preserving reprocessing can be found at www.ak-i.org, link to "AKI-Brochures", "Red brochure".
General safety information To prevent damage caused by improper setup or operation, and to not compromise the manufacturer warranty and liability: ► Use the product only according to these instructions for use. ► Follow the safety and maintenance instructions. ► Ensure that the product and its accessories are operated and used only by persons with the requisite training, knowledge and experience. ► Store any new or unused products in a dry, clean, and safe place. ► Prior to use, check that the product is in good working order. ► Keep the instructions for use accessible for the user. Note The user is obligated to report all severe events in connection with the product to the manufacturer and the responsible authorities of the state in which the user is located. Notes on surgical procedures It is the user's responsibility to ensure that the surgical procedure is performed correctly. Appropriate clinical training as well as a theoretical and practical proficiency of all the required operating techniques, including the use of this product, are prerequisites for the successful use of this product. The user is required to obtain information from the manufacturer if there is an unclear preoperative situation regarding the use of the product.
3.2
General information
3.3
Reusable products
Influences of the reprocessing which lead to damage to the product are not known. A careful visual and functional inspection before the next use is the best opportunity to recognize a product that is no longer functional, see Inspection.
3.8.1
Manual cleaning with immersion disinfection
Phase
Step
T [°C/°F]
t [min]
Conc. [%]
Water quality
Chemical
I
Disinfecting cleaning
RT (cold)
>15
2
D–W
► If applicable, rinse non-visible surfaces preferably with deionized water, with a disposable syringe for example. ► Remove any visible surgical residues to the extent possible with a damp, lint-free cloth. ► Transport the dry product in a sealed waste container for cleaning and disinfection within 6 hours.
Aldehyde-free, phenol-free, and QUAT-free concentrate, pH ~ 9*
II
Intermediate rinse
RT (cold)
1
-
D–W
-
3.5
III
Disinfection
RT (cold)
5
2
D–W
Aldehyde-free, phenol-free, and QUAT-free concentrate, pH ~ 9*
IV
Final rinse
RT (cold)
1
-
FD-W
-
V
Drying
RT
-
-
-
-
3.4
Preparations at the place of use
Preparing for cleaning
► Disassemble the product immediately after use, as described in the respective instructions for use. ► Disassemble the product prior to cleaning, see Disassembly.
Note During cleaning the clamping sleeve 7 must sit loosely on the instrument shaft. Note To ensure proper cleaning of spacers 9, the PEEK forks must sit loosely (so that they can be turned in both directions) on the thread.
3.6
Disassembly
► Uncouple spacer 9 from insertion instrument 2 by pressing "push to clean" button 5 at the handle of insertion
D–W: Drinking water FD–W: Fully desalinated water (demineralized, microbiological, at least of drinking water quality) RT: Room temperature *Recommended: BBraun Stabimed fresh
instrument 2 and simultaneously pulling it out, axially, towards the working end. ► If applicable, unscrew repositioning instrument 3 from insertion instrument 2 by turning it counterclockwise. ► Before reprocessing, ensure that the clamping sleeve 7 is sitting loosely on the instrument shaft.
► Note the information on appropriate cleaning brushes and disposable syringes, see Validated cleaning and dis-
3.7
Cleaning/Disinfection
3.7.1
Product-specific safety information on the reprocessing method
Phase I ► Fully immerse the product in the cleaning/disinfectant for at least 15 min. Ensure that all accessible surfaces are moistened. ► Clean the product with a suitable cleaning brush in the solution until all discernible residues have been removed from the surface. ► If applicable, brush through non-visible surfaces with an appropriate cleaning brush for at least 1 min. ► Mobilize non-rigid components, such as set screws, links, etc. during cleaning. ► Thoroughly rinse through these components with the cleaning disinfectant solution (at least five times), using a disposable syringe.
Damage to or destruction of the product due to inappropriate cleaning/disinfecting agents and/or excessive temperatures! ► Following the manufacturer's instructions, use cleaning and disinfecting agents – that are approved for e.g., aluminum, plastics, high-grade steel, – that do not attack softeners (e.g., in silicone). ► Observe specifications regarding concentration, temperature and exposure time. ► Do not exceed the maximum allowable disinfection temperature of 95 °C.
3.7.2
Validated cleaning and disinfection procedure
Validated procedure
Specific requirements
Reference
Manual cleaning with immersion disinfection
■ Cleaning brush: 250 mm/∅ 3.7 mm,
Chapter Manual cleaning/disinfection and subsection:
■ FW867R ■ FW945R
■ Chapter Manual cleaning with ■ Disposable syringe 20 ml immersion disinfection ■ When cleaning instruments with mov-
e.g. GK469200
able hinges, ensure that these are in an open position and, if applicable, move the hinge while cleaning. medical compressed air
■ FW868R
■ Cleaning brush: 250 mm/∅ 3.7 mm, e.g. GK469200
Phase II ► Rinse/flush the product thoroughly (all accessible surfaces) under running water. ► Mobilize non-rigid components, such as set screws, joints, etc. during rinsing. ► Drain any remaining water fully. Phase III ► Fully immerse the product in the disinfectant solution. ► Mobilize non-rigid components, such as set screws, joints, etc. during rinsing. ► Rinse lumens at least 5 times at the beginning of the exposure time using an appropriate disposable syringe. Ensure that all accessible surfaces are moistened. Phase IV ► Rinse/flush the product thoroughly (all accessible surfaces). ► Mobilize non-rigid components, such as set screws, joints, etc. during final rinse. ► Rinse lumens with an appropriate disposable syringe at least five times. ► Drain any remaining water fully.
■ Drying phase: Use a lint-free cloth or Manual cleaning with ultrasound and immersion disinfection
infection procedure.
Chapter Manual cleaning/disinfection and subsection:
■ Chapter Manual cleaning with ■ Disposable syringe 20 ml ultrasound and immersion dis■ Keep working ends open for cleaning. infection ■ When cleaning instruments with movable hinges, ensure that these are in an open position and, if applicable, move the hinge while cleaning.
■ Drying phase: Use a lint-free cloth or
Phase V ► Dry the product in the drying phase with suitable equipment (e.g. cloth, compressed air), see Validated cleaning and disinfection procedure.
3.8.2
Manual cleaning with ultrasound and immersion disinfection
Phase
Step
T [°C/°F]
t [min]
Conc. [%]
Water quality
Chemical
I
Ultrasonic cleaning
RT (cold)
>15
2
D–W
Aldehyde-free, phenol-free, and QUAT-free concentrate, pH ~ 9*
II
Intermediate rinse
RT (cold)
1
-
D–W
-
III
Disinfection
RT (cold)
5
2
D–W
Aldehyde-free, phenol-free, and QUAT-free concentrate, pH ~ 9*
IV
Final rinse
RT (cold)
1
-
FD-W
-
V
Drying
RT
-
-
-
-
medical compressed air Mechanical alkaline cleaning and thermal disinfection
■ FW867R ■ FW945R
■ Place the product on a tray that is suitable for cleaning (avoid rinsing blind spots).
Chapter Mechanical cleaning/disinfection and subsection:
■ Chapter Mechanical alkaline
■ Connect components with lumens and
cleaning and thermal disinfecting
channels directly to the rinsing port of the injector carriage.
■ To flush the product: Use a flushing nozzle or flushing sleeve.
■ Place the product on the tray with all product links and joints open. Manual pre-cleaning with ultrasound and brush, and subsequent mechanical alkaline cleaning and thermal disinfection
■ FW868R
■ Cleaning brush: 250 mm/∅ 3.7 mm, e.g. GK469200
Chapter Mechanical cleaning/disinfection with manual pre-cleaning and subsection:
■ Disposable syringe 20 ml ■ Chapter Manual pre-cleaning ■ When cleaning instruments with movwith ultrasound and brush able hinges, ensure that these are in an open position and, if applicable, move the joint while cleaning.
■ Chapter Mechanical alkaline
■ Place the product on a tray that is
cleaning and thermal disinfecting
suitable for cleaning (avoid rinsing blind spots).
■ Connect components with lumens and channels directly to the rinsing port of the injector carriage.
■ To flush the product: Use a flushing nozzle or flushing sleeve.
■ Place the product on the tray with all product links and joints open.
3.8
Manual cleaning/disinfection
► Prior to manual disinfecting, allow water to drip off for a sufficient length of time to prevent dilution of the dis-
infecting solution. ► After manual cleaning/disinfection, check visible surfaces visually for residues. ► Repeat the cleaning/disinfection process if necessary.
D–W: FD–W:
Drinking water Fully desalinated water (demineralized, low microbiological contamination: drinking water quality at least) RT: Room temperature *Recommended: BBraun Stabimed fresh ► Note the information on appropriate cleaning brushes and disposable syringes, see Validated cleaning and dis-
infection procedure. Phase I ► Clean the product in an ultrasonic cleaning bath (frequency 35 kHz) for at least 15 min. Ensure that all accessible surfaces are immersed and acoustic shadows are avoided. ► Clean the product with a suitable cleaning brush in the solution until all discernible residues have been removed from the surface. ► If applicable, brush through non-visible surfaces with an appropriate cleaning brush for at least 1 min. ► Mobilize non-rigid components, such as set screws, links, etc. during cleaning. ► Thoroughly rinse through these components with the cleaning disinfectant solution (at least five times), using a disposable syringe. Phase II ► Rinse/flush the product thoroughly (all accessible surfaces) under running water. ► Mobilize non-rigid components, such as set screws, joints, etc. during rinsing. ► Drain any remaining water fully. Phase III ► Fully immerse the product in the disinfectant solution. ► Mobilize non-rigid components, such as set screws, joints, etc. during rinsing. ► Rinse lumens at least five times at the beginning of the exposure time with an appropriate disposable syringe. Ensure that all accessible surfaces are moistened. Phase IV ► Rinse/flush the product thoroughly (all accessible surfaces) under running water. ► Mobilize non-rigid components, such as set screws, joints, etc. during final rinse. ► Rinse lumens with an appropriate disposable syringe at least five times. ► Drain any remaining water fully.
Phase V ► Dry the product in the drying phase with suitable equipment (e.g. cloth, compressed air), see Validated cleaning and disinfection procedure.
3.9
Mechanical cleaning/disinfection
Note The cleaning and disinfection device used for processing must be serviced and checked at regular intervals.
Mechanical alkaline cleaning and thermal disinfecting
Machine type: single-chamber cleaning/disinfecting machine without ultrasound Phase
Step
D [°C/°F]
I
Prerinse
<25/77
II
Cleaning
55/131
t [min]
► Allow the product to cool down to room temperature. ► Dry the product if it is wet or damp.
3.11.1 Visual inspection
Note The cleaning and disinfection device must be of tested and approved effectiveness (e.g. FDA approval or CE mark according to DIN EN ISO 15883).
3.9.1
3.11 Inspection
Water quality
Chemical/Note
3
D–W
-
10
FD-W
■ Concentrate, alkaline: – pH ~ 13 – <5 % anionic surfactant
► Ensure that all soiling has been removed. In particular, pay attention to mating surfaces, hinges, shafts, recessed
areas, drill grooves and the sides of the teeth on rasps. ► If the product is dirty: repeat the cleaning and disinfection process. ► Check the product for damage, e.g. insulation or corroded, loose, bent, broken, cracked, worn or severely
scratched and fractured components. ► Check the product for missing or faded labels. ► Check the products with long, slim shapes (in particular rotating instruments) for deformities. ► Check the product for damage to the spiral element. ► Check the cutting edges for continuity, sharpness, nicks and other damage. ► Check the surfaces for rough spots. ► Check the product for burrs that could damage tissue or surgical gloves. ► Check the product for loose or missing parts. ► Immediately put aside damaged or inoperative products and send them to Aesculap Technical Service, see Tech-
nical service.
3.11.2 Functional test
III
Intermediate rinse
>10/50
1
FD-W
-
► Assemble disassembled products, see Assembly. ► Check that the product functions correctly. ► Check that all moving parts are working property (e.g. hinges, locks/latches, sliding parts etc.). ► Check for compatibility with associated products. ► Immediately put aside inoperative products and send them to Aesculap Technical Service, see Technical service.
IV
Thermal disinfecting
90/194
5
FD-W
-
3.12 Assembly
V
Drying
-
-
-
According to the program for cleaning and disinfection device
■ working solution 0.5% – pH = 11*
DW: FD–W:
Drinking water Fully desalinated water (demineralized, low microbiological contamination: drinking water quality at least) *Recommended: BBraun Helimatic Cleaner alcaline
Note Account for the size of the implant when selecting the insertion instrument! ► Remove irrigation connector 4 from the handle end of insertion instrument 2. ► Verify that clamping sleeve 7 on insertion instrument 2 has been loosened. ► Turn clamping sleeve 7 counterclockwise until it is no longer in connection with connecting piece 8 of insertion
instrument 2. ► Connect the spacer 9 corresponding to the intraoperatively selected implant height to the insertion
instrument 2. ► Insert spacer 9 in the hole of the connecting piece, observing the "CRANIAL" and "CAUDAL" markings on the
► Check visible surfaces for residues after mechanical cleaning/disinfecting.
3.10 Mechanical cleaning/disinfection with manual pre-cleaning Note The cleaning and disinfection device must be of tested and approved effectiveness (e.g. FDA approval or CE mark according to DIN EN ISO 15883). Note The cleaning and disinfection device used for processing must be serviced and checked at regular intervals.
3.10.1 Manual pre-cleaning with ultrasound and brush Phase
Step
D [°C/°F]
t [min]
Conc. [%]
Water quality
Chemical
I
Ultrasonic cleaning
RT (cold)
>15
2
D–W
Aldehyde-free, phenol-free, and QUAT-free concentrate, pH ~ 9*
II
Rinsing
RT (cold)
1
-
D–W
-
infection procedure. Phase I ► Clean the product in an ultrasonic cleaning bath (frequency 35 kHz) for at least 15 min. Ensure that all accessible surfaces are immersed and acoustic shadows are avoided. ► Clean the product with a suitable cleaning brush in the solution until all discernible residues have been removed from the surface. ► If applicable, brush through non-visible surfaces with an appropriate cleaning brush for at least 1 min. ► Mobilize non-rigid components, such as set screws, links, etc. during cleaning. ► Thoroughly rinse through these components with the cleaning disinfectant solution (at least five times), using a disposable syringe. Phase II ► Rinse/flush the product thoroughly (all accessible surfaces) under running water. ► Mobilize non-rigid components, such as set screws, joints, etc. during rinsing.
3.10.2 Mechanical alkaline cleaning and thermal disinfecting Machine type: single-chamber cleaning/disinfection device without ultrasound T [°C/°F]
I
Prerinse
<25/77
II
Cleaning
55/131
► Appropriately protect products with fine working tips. ► Store products with ratchet locks fully opened or locked no further than in the first notch. ► Place the product in its holder or on a suitable tray. Ensure that sharp edges are covered. ► Package trays appropriately for the sterilization process (e.g. in Aesculap sterile containers). ► Ensure that the packaging provides sufficient protection against contamination of the product during storage.
3.14 Steam sterilization Note The product may only be sterilized in disassembled condition. ► Check to ensure that the sterilizing agent will come into contact with all external and internal surfaces (e.g., by
opening any valves and faucets).
► Note the information on appropriate cleaning brushes and disposable syringes, see Validated cleaning and dis-
Step
3.13 Packaging
► Validated sterilization process
D–W: Drinking water RT: Room temperature *Recommended: BBraun Stabimed fresh
Phase
spacer 9 and insertion instrument 2. At the same time keep button 5, "push to clean", at the handle of insertion instrument 2 pressed down. ► Insert spacer 9 down to the stop in insertion instrument 2. ► Release "push to clean" button 5. By axially pulling at spacer 9, check that the spacer is properly locked in insertion instrument 2. ► Turn adjusting wheel 6 counterclockwise to set the depth stop to minimum insertion depth.
t [min]
Water quality
Chemical
3
D–W
-
10
FD-W
■ Concentrate, alkaline: – pH = 13 – <5 % anionic surfactant
■ working solution 0.5% – pH = 11* III
Intermediate rinse
>10/50
1
FD-W
-
IV
Thermal disinfecting
90/194
5
FD–W
-
V
Drying
-
-
-
According to the program for cleaning and disinfection device
D–W: FD–W:
Drinking water Fully desalinated water (demineralized, low microbiological contamination: drinking water quality at least) *Recommended: BBraun Helimatic Cleaner alcaline ► Check visible surfaces for residues after mechanical cleaning/disinfecting.
– Disassemble the product – Steam sterilization using fractional vacuum process – Steam sterilizer according to DIN EN 285 and validated according to DIN EN ISO 17665 – Sterilization using fractionated vacuum process at 134 °C/holding time 5 min ► If several devices are sterilized at the same time in the same steam sterilizer: Ensure that the maximum permitted load according to the manufacturers’ specifications is not exceeded.
3.15 Storage ► Store sterile products in germ-proof packaging, protected from dust, in a dry, dark, temperature-controlled area.
4.
Technical service
CAUTION Modifications carried out on medical technical equipment may result in loss of guarantee/warranty rights and forfeiture of applicable licenses. ► Do not modify the product. ► For service and repairs, please contact your national B. Braun/Aesculap agency. Service addresses Aesculap Technischer Service Am Aesculap-Platz 78532 Tuttlingen / Germany Phone: +49 7461 95-1601 Fax: +49 7461 16-2887 E-Mail: [email protected] Other service addresses can be obtained from the address indicated above.
5.
Disposal
WARNING Risk of infection due to contaminated products! ► Adhere to national regulations when disposing of or recycling the product, its components and its packaging. WARNING Risk of injury due to sharp-edged and/or pointed products! ► When disposing of or recycling the product, ensure that the packaging prevents injury by the product. Note The user institution is obliged to reprocess the product before its disposal, see Validated reprocessing procedure. TA011997
2021-05
V6
Change No. AE0060911