BBraun
Combined neck and head rest FF140
27 Pages
Preview
Page 1
Aesculap® 1
FF143 2
3
FF141 4
Aesculap Spine
5 en USA
de fr es it pt nl sv ru cs pl sk tr
Instructions for use/Technical description Combined neck and head rest FF140 Note for U.S. users This Instructions for Use is NOT intended for United States users. Please discard. The Instructions for Use for United States users can be obtained by visiting our website at www.aesculapImplantsystems.com. If you wish to obtain a paper copy of the Instructions for Use, you may request one by contacting your local Aesculap representative or Aesculap's customer service at 1-866-229-3002. A paper copy will be provided to you upon request at no additional cost. Gebrauchsanweisung/Technische Beschreibung Kombinierte HWS-Kopfstütze FF140 Mode d’emploi/Description technique Appui-tête et cou combiné FF140 Instrucciones de manejo/Descripción técnica Reposacabezas con reposanucas FF140 Istruzioni per l’uso/Descrizione tecnica Poggiatesta combinato FF140 Instruções de utilização/Descrição técnica Apoio combinado para cabeça e pescoço FF140 Gebruiksaanwijzing/Technische beschrijving Gecombineerde nek- en hoofdsteun FF140 Bruksanvisning/Teknisk beskrivning Kombinerat hals- och huvudstöd FF140 Инструкция по примению/Техническое описание Комбинированный подголовник HWS FF140 Návod k použití/Technický popis Kombinovaná opěrka krku a hlavy FF140 Instrukcja użytkowania/Opis techniczny Łączona podpora głowy i odcinka szyjnego kręgosłupa FF140 Návod na použitie/Technický opis Kombinovaná opierka hlavy a krku FF140 Kullanım Kılavuzu/Teknik açiklama Kombine baş ve boyun desteği FF140
6
Aesculap® – a B. Braun brand TA007106
2020-05
V6
Change No. 62702
12
7 8
13 13
FF144
9 10
13
14
1
Aesculap AG | Am Aesculap-Platz | 78532 Tuttlingen | Germany Phone +49 (0) 7461 95-0 | Fax +49 (0) 7461 95-26 00 | www.aesculap.com
11
14
en
2.3 ®
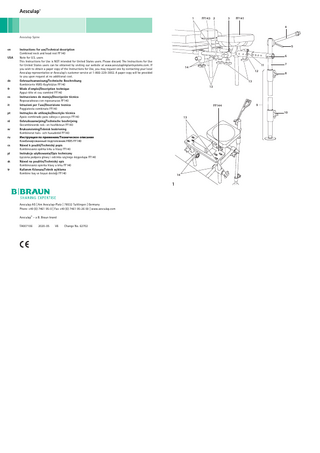
Aesculap Combined neck and head rest FF140 Legend 1 Fastening rods 2 Star-knob screw for neck support bench 3 Star-knob screw for headrest 4 Rope support face with guide pulley 5 Screw for fixing the rope support face 6 Bolt 7 Connecting piece with rope support face 8 Star-knob screw for stand 9 Stand 10 Star-knob screw for height adjustment 11 Spring cotter pin 12 Bolt 13 Star-knob screws for fastening rods 14 Frame connecting pieces
1.
About this document
Note General risk factors associated with surgical procedures are not described in these instructions for use.
1.1
Scope
These instructions for use apply for the following products: Art. no.
Designation
FF140
Combined neck and head rest
Application
WARNING Risk of injury and/or malfunction! ► Prior to each use, inspect the product for loose, bent, broken, cracked, worn, or fractured components. ► Always carry out a function test prior to each use of the product. WARNING Risk of infection! ► Cover the head cushion FF141, rubber band FF142 and neck cushion FF143 with surgical drape. CAUTION Damage to the product due to inappropriate cleaning/disinfecting agents! ► Only use cleaning/disinfecting agents approved for surface cleaning. Follow the manufacturer’s instructions for the respective cleaning/disinfecting agent. CAUTION Damage to the product due to inappropriate cleaning/disinfecting agents! ► Use cleaning and disinfecting agents according to the manufacturer’s instructions which – be approved for plastic material and high-grade steel, – do not attack softeners (e.g. in silicone). ► Observe specifications regarding concentration, temperature and exposure time.
3.
Validated reprocessing procedure
3.1
General safety notes
Note Adhere to national statutory regulations, national and international standards and directives, and local, clinical hygiene instructions for reprocessing. Note For patients with Creutzfeldt-Jakob disease (CJD), suspected CJD, or possible variants of CJD, observe the relevant national regulations concerning the reprocessing of products.
Note The applicable CE mark for the product can be seen on the label or packaging of the product.
Note It should be noted that successful reprocessing of this medical device can only be guaranteed following prior validation of the reprocessing method. The operator/reprocessing technician is responsible for this.
► For article-specific instructions for use as well as information on material compatibility and lifetime see B. Braun
3.2
eIFU at eifu.bbraun.com
1.2
Safety messages
Safety messages make clear the dangers to patient, user and/or product that could arise during the use of the product. Safety messages are labeled as follows: WARNING Indicates a possible threat of danger. If not avoided, minor or moderate injury may result. CAUTION Indicates a possible threat of material damage. If not avoided, the product may be damaged.
2.
Clinical use
2.1
Areas of use and limitations of use
2.1.1
Intended use
The combined neck and head rest is used within various surgical procedures to keep the head in a fixed position. The combined neck and head rest permits adaptation to the individual patient and the best possible access to the surgical site, particularly for intra-operative C-arm fluoroscopy.
2.1.2
Reusable products
Influences of the reprocessing which lead to damage to the product are not known. A careful visual and functional inspection before the next use is the best opportunity to recognize a product that is no longer functional, see Inspection.
3.3
Preparation before cleaning
► Remove any visible surgical residues as much as possible with a damp, lint-free cloth. ► Carry out non-fixating/NaCl-free pre-cleaning immediately after use.
3.4
Wipe disinfection
Phase
Step
T [°C/°F]
t [min]
Conc. [%]
Water quality
Chemical
I
Cleaning
RT
1
-
-
17 % Propan-1-ol, 0.23 % Didecyldimethylammonium chloride*
II
Wipe disinfection
RT
≥1
-
-
17 % Propan-1-ol, 0.23 % Didecyldimethylammonium chloride*
Indications
Note The manufacturer is not responsible for any use of the product against the specified indications and/or the described applications.
RT: Room temperature *Recommended: Meliseptol® Wipes sensitive (B. Braun)
For indications, see Intended use.
Phase I ► Remove any visible residues with a disposable disinfectant wipe.
2.1.3
Contraindications
2.2
Safety information
Phase II ► Wipe all surfaces of the optically clean product with a fresh, disposable disinfectant wipe. ► Observe contact time requirements (at least 1 min).
2.2.1
Clinical user
3.5
Inspection
3.5.1
Visual inspection
No known contraindications.
General safety information To prevent damage caused by improper setup or operation, and to not compromise the manufacturer warranty and liability: ► Use the product only according to these instructions for use. ► Follow the safety and maintenance instructions. ► Ensure that the product and its accessories are operated and used only by persons with the requisite training, knowledge and experience. ► Store any new or unused products in a dry, clean, and safe place. ► Prior to use, check that the product is in good working order. ► Keep the instructions for use accessible for the user. Note The user is obligated to report all severe events in connection with the product to the manufacturer and the responsible authorities of the state in which the user is located. Notes on surgical procedures It is the user's responsibility to ensure that the surgical procedure is performed correctly. Appropriate clinical training as well as a theoretical and practical proficiency of all the required operating techniques, including the use of this product, are prerequisites for the successful use of this product. The user is required to obtain information from the manufacturer if there is an unclear preoperative situation regarding the use of the product.
2.2.2
Product
Product-specific safety information Non-compliance with the following rules will result in complete exclusion of liability on the part of Aesculap.
2.2.3
Sterility
The product is delivered in an unsterile condition. ► Clean the new product after removing its transport packaging and prior to its initial sterilization.
► If the product is dirty: repeat the cleaning and disinfection process. ► Check the product for damage, e.g. insulation or corroded, loose, bent, broken, cracked, worn or severely
scratched and fractured components. ► Check the product for missing or faded labels. ► Check the products with long, slim shapes (in particular rotating instruments) for deformities. ► Check the product for damage to the spiral element. ► Check the cutting edges for continuity, sharpness, nicks and other damage. ► Check the surfaces for rough spots. ► Check the product for burrs that could damage tissue or surgical gloves. ► Check the product for loose or missing parts. ► Immediately put aside damaged or inoperative products and send them to Aesculap Technical Service, see Tech-
nical service.
3.5.2
Functional test
► Assemble disassembled products, see Assembly. ► Check that the product functions correctly. ► Check that all moving parts are working property (e.g. hinges, locks/latches, sliding parts etc.). ► Check for compatibility with associated products. ► Immediately put aside inoperative products and send them to Aesculap Technical Service, see Technical service.
3.6
Assembly
Note The combined neck and head rest FF140 is supplied partly assembled.
3.6.1
Assembly of the head rest
► Adjust the width of the fastening rods 1 by loosening the star-knob screws 13. ► Insert the fastening rods 1 into the dedicated recess in the operating table and tighten. ► Fit connecting piece with rope support face 7 onto the stand 9 and tighten the star-knob screw 8. ► Connect the entire stand 9 to the head rest and secure using the spring cotter pin 11 and bolt 12. ► Guide the rope support face 4 to the desired position by loosening the screw 5. ► Adjust the length of the stand by loosening the star-knob button 10.
Note If the height of the operating table is changed at a later point in time, the length of the stand will need re-adjusting.
3.6.2
Assembly of cushions
► Affix the neck cushion FF143 to the neck support bench with Velcro. ► Adjust the height of the neck support bench by loosening the star-knob screw 2. ► Place the head cushion FF141 onto the headrest and secure using perforated rubber band (mushroom-shaped
bolts are provided on the underside of the headrest for this purpose). ► Adjust the height of the headrest by loosening the star-knob screw 3.
3.6.3
Assembly of the x-ray-transparent lumbar support FF144 (optional)
► Loosen the star-knob screws 13 on the frame connecting pieces 14. ► Remove the transverse bar. ► Tilt the frame connecting pieces 14 90° outward, see Fig. 1. ► Insert the lumbar support FF144 and tighten star-knob screw 13.
3.7
Packaging
► Ensure that the packaging provides sufficient protection against contamination of the product during storage.
3.8
Storage
► Store sterile products in germ-proof packaging, protected from dust, in a dry, dark, temperature-controlled area.
4.
Maintenance and service
4.1
Maintenance
For technical service, please contact your national B. Braun/Aesculap agency, see Technical service.
4.2
Technical service
CAUTION Modifications carried out on medical technical equipment may result in loss of guarantee/warranty rights and forfeiture of applicable licenses. ► Do not modify the product. ► For service and repairs, please contact your national B. Braun/Aesculap agency. Service addresses Aesculap Technischer Service Am Aesculap-Platz 78532 Tuttlingen / Germany Phone: +49 7461 95-1601 Fax: +49 7461 16-2887 E-Mail: [email protected] Other service addresses can be obtained from the address indicated above.
5.
Disposal
WARNING Risk of infection due to contaminated products! ► Adhere to national regulations when disposing of or recycling the product, its components and its packaging. WARNING Risk of injury due to sharp-edged and/or pointed products! ► When disposing of or recycling the product, ensure that the packaging prevents injury by the product. Note The user institution is obliged to reprocess the product before its disposal, see Validated reprocessing procedure. TA007106
2020-05
V6
Change No. 62702