BBraun
Rigid autoclavable endoscopes
42 Pages
Preview
Page 1
Aesculap®
Aesculap Endoscopic Technology
Instructions for use/Technical description Rigid autoclavable endoscopes Instructions for use/Technical description Rigid autoclavable endoscopes Note for U.S. users This Instructions for Use is NOT intended for United States users. Please discard. The Instructions for Use for United States users can be obtained by visiting our website at www.aesculapusa.com and clicking the "Products" menu. If you wish to obtain a paper copy of the Instructions for Use, you may request one by contacting your local Aesculap representative or Aesculap's customer service at 1-800-282-9000. A paper copy will be provided to you upon request at no additional cost. Gebrauchsanweisung/Technische Beschreibung Starre autoklavierbare Endoskope Mode d’emploi/Description technique Endoscopes rigides autoclavables Instrucciones de manejo/Descripción técnica Endoscopios rígidos autoclavables Istruzioni per l’uso/Descrizione tecnica Endoscopi rigidi autoclavabili Instruções de utilização/Descrição técnica Endoscópios rígidos esterilizáveis em autoclave Gebruiksaanwijzing/Technische beschrijving Stijve, in de autoclaaf steriliseerbare endoscopen Bruksanvisning/Teknisk beskrivning Stela autoklaverbara endoskop Инструкция по примению/Техническое описание Жесткие автоклавируемые эндоскопы Návod k použití/Technický popis Rigidní autoklávovatelné endoskopy Instrukcja użytkowania/Opis techniczny Nadające się do autoklawowania endoskopy sztywne Návod na použitie/Technický opis Tuhé autoklávovateľné endoskopy Kullanım Kılavuzu/Teknik açiklama Sağlam otomatik ayarlanabilir endoskop 사용 설명서 / 기술 설명 의료용내시경 (Wide-Angle Optic / 형명개별기재 )
3
4 5
6
2
1
16 1
2
1
2
7
3 4
5
16
7
6
16 3 5 4
7
10 9 6
6
8 16 Aesculap AG | Am Aesculap-Platz | 78532 Tuttlingen | Germany Phone +49 (0) 7461 95-0 | Fax +49 (0) 7461 95-26 00 | www.aesculap.com Aesculap – a B. Braun company TA-Nr. 011057
2018-01
Änd.-Nr. 58216
3 - DIR 93/42/EEC
6
5 V6
1
4
7
2
11
16 15
12
13
14
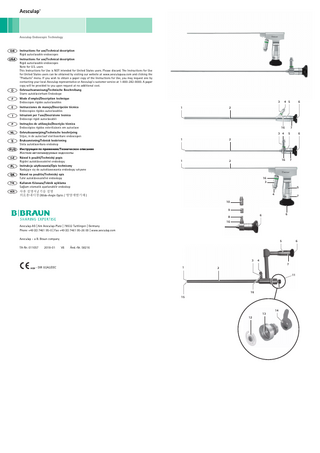
Safe handling and preparation Aesculap® Rigid autoclavable endoscopes Legend
This manual contains instructions for the preparation, reprocessing, and disposal of the endoscope. It does not contain information on the actual application of the endoscope. Risk of injury from defective endoscopes! ► Only use an endoscope if it is in perfect condition. WARNING
1 Distal window 2 Sheath 3 Optical cable connection 4 Illumination surface of the optical cable connection 5 Eyepiece housing 6 Eyepiece window 7 Inscription ring 8 ACMI adapter (fixed) 9 Wolf adapter 10 Adapter Aesculap, Olympus, Storz 11 Valve at the working channel 12 Silicone flap valve 13 Valve body 14 Sealing cap 15 Working channel 16 Color ring
► Ensure that the product and its accessories are operated and used only by persons with the requisite training,
knowledge, or experience. ► Read, follow, and keep the instructions for use. ► Use the product only in accordance with its intended use, see Intended use. ► Remove the transport packaging and clean the new product, either manually or mechanically, prior to its initial
sterilization. ► Store any new or unused products in a dry, clean, and safe place. ► Prior to each use, inspect the product for loose, bent, broken, cracked, worn, or fractured components. ► Do not use the product if it is damaged or defective. Set aside the product if it is damaged. ► Replace any damaged components immediately with original spare parts. ► To avoid damage to the working end: Carefully insert the product through the working channel (e.g. trocar). ► Make certain a reserve product is available. ► Do not, under any circumstances, put down the product on the patient or on the surgical drape covering the
patient.
Safe operation
Symbols on product and packages Caution, general warning symbol Caution, see documentation supplied with the product
Risk of injury and/or malfunction! ► Always carry out a function check prior to using the product. WARNING Risk of injury when using the product beyond the field of view! ► Apply the product only under visual control.
Date of manufacture WARNING Product supplied in unsterile condition
Steam sterilization at temperatures up to 134 °C
Function checks ► Check the optical function of the endoscope. The image has to be clear and distinct. ► Ensure that the distal window 1, the eyepice window 6 and the illumination surface 4 of the optical cable
connection 3 are not cloudy, dirty or scratched. ► Hold optical cable connection 3 of the endoscope against the light and check that the light fibers at the distal
end light up evenly. ► Inspect the sheath for dents, bends, and scratches.
Low temperature and plasma sterilization
Use of the endoscope Symbol indicating High-Definition technology WARNING
Color coding The viewing direction of the endoscope is indicated by color ring 16 at optical cable connection 3. Color
Viewing direction
Green
0°
Black
12°
Black
45°
Red
30°
Yellow
70°
WARNING
Risk of burns to patient and user, caused by high-intensity light! ► Make certain the distal end of the endoscope or optical cable connection does not touch human tissue or any flammable or heat-sensitive materials while the light source is active. ► Do not put down the endoscope on the patient. ► Do not touch the distal end of the endoscope and the optical cable connection. ► Adjust the light source to the minimum required power for optimal illumination of the endoscopic image. ► Only use light sources of a power rating of up to 300 W. Burns caused by high-frequency current (HF current)! ► When using an endoscope and HF electrodes at the same time, be cautious to activate the HF current under visual control only. ► Ensure that the active electrode is never in the immediate vicinity of electrically conductive components (e.g. trocar, endoscope).
CAUTION
Damage to the endoscope by bending the endoscope sheath! ► Do not bend the endoscope sheath. ► Use endoscopes with their appropriate sheaths and working trocars only. ► Always hold the endoscope at the eyepiece housing but never at the sheath.
CAUTION
Damage to the flap valve caused by instruments in endoscopes with a working channel! ► To avoid damage to the valve, apply proper caution when introducing sharp or pointed instruments.
Applicable to ► For item-specific instructions for use and information on material compatibility, see also the Aesculap Extranet
at https://extranet.bbraun.com
Intended use Rigid autoclavable endoscopes are used for visualizing body cavities. They may be used only for the respective approved or specified intended applications: ■ Arthroscopy: Endoscopes for arthroscopy, to visualize joints and tissues – PE182A, PE202A, PE484A, PE485A, PE505A, PE514A, PE525A ■ Hysteroscopy: Endoscopes for hysteroscopy, to visualize the uterus and the tube entrances – PE218A, PE508A, PE528A ■ Cystoscopy: Endoscopes for cystoscopy, to visualize the urethra and the urinary bladder – PE508A, PE522A, PE528A, PE530A ■ Otolaryngology: Endoscopes for otolaryngology, to visualize tissues in the ear, nose and throat area – PE185A, PE485A, PE505A, PE525A ■ Laparoscopy and thoracoscopy: Endoscopes for visualizing tissue, blood vessels and organs in the abdominal and thoracic cavities – PE590A, PE610A, PE889A, PE898A, PE909A, PE969A, PE970A ■ Neuroendoscopy: Endoscopes for neuroendoscopy, to visualize the nasal structures, the adjacent skull base, the pituitary gland and adjacent structures, for transnasal approach – PE487A, PE507A
Available sizes The rigid autoclavable endoscopes are available in the following sizes and designs: ■ Sheath diameter 2-10 mm ■ Straight endoscopes ■ Angled endoscopes ■ Endoscopes with a working channel
► Use endoscopes only with halogen light sources equipped with a spare lamp or with xenon light sources. ► Observe the respective manufacturer’s instructions when using the endoscope in combination with other equip-
ment, instruments, or optical cables. ► When using the endoscope in combination with any electromedical device, make sure that the respective BF con-
ditions are adhered to (insulated, floating patient application piece).
Disassembling ► Unscrew adapter 9 or 10, respectively, from the endoscope. ► For endoscopes with a working channel:
– Remove sealing cap 14. – Unscrew valve body 13. – Remove the silicone flap valve 12.
Assembling ► Screw on adapter 9 or 10, respectively. ► For endoscopes with a working channel:
– Insert silicone flap valve 12. – Screw on valve body 13. – Press on the sealing cap 14.
Validated reprocessing procedure
Manual cleaning with immersion disinfection Phase
Step
T [°C/°F]
t [min]
Conc. [%]
Water quality
Chemicals
I
Clean
35-45/ 95-113
5
0,8
D–W
Cidezyme/Enzol
II
Intermediate rinse
RT (cold)
3x1
-
D–W
-
III
Disinfection
20-25/ 68-77
12
0,55
D–W
Cidex OPA (process solution, 0.55 % ortho-phthalaldehyde)
IV
Final rinse
RT (cold)
3x2
-
FD–W (sterile)
-
V
Dry
RT
-
-
-
-
General safety notes Note Adhere to national statutory regulations, national and international standards and directives, and local, clinical hygiene instructions for reprocessing. Note For patients with Creutzfeldt-Jakob disease (CJD), suspected CJD, or possible variants of CJD, observe the relevant national regulations concerning the reprocessing of products. Note Mechanical reprocessing should be favored over manual cleaning as it gives better and more reliable results. Note It should be noted that successful reprocessing of this medical device can only be guaranteed following prior validation of the reprocessing method. The operator/reprocessing technician is responsible for this. Note If there is no final sterilization, then a virucidal disinfectant must be used. Note For up-to-date information about reprocessing and material compatibility, see also the Aesculap Extranet at www.aesculap-extra.net Note Only clean medical products can be sterilized safely and effectively. Cleaning is therefore of special importance in the reprocessing procedure. Rigid autoclavable endoscopes are delivered in unsterile condition. ► The endoscopes must be cleaned and sterilized before being used. Endoscopes are delicate optical devices. Aesculap therefore recommends reprocessing them separately. For sterilization, storage and sterile setup of endoscopes, Aesculap offers special optics trays.
General information Dried or affixed surgical residues can make cleaning more difficult or ineffective and lead to corrosion. Therefore the time interval between application and processing should not exceed 6 h; also, neither fixating pre-cleaning temperatures >45 °C nor fixating disinfecting agents (active ingredient: aldehydes/alcohols) should be used. Excessive dosages of neutralizing agents or basic cleaners may result in a chemical attack and/or fading and the laser marking on stainless steel becoming unreadable either visually or by machine. Residues containing chlorine or chlorides, e.g., in surgical residues, medicines, saline solutions, and in the service water used for cleaning, disinfection, and sterilization, will cause corrosion damage (pitting, stress corrosion) and result in the destruction of stainless steel products. These must be removed by rinsing thoroughly with demineralized water and then drying. Perform additional drying, if necessary. Only process chemicals that have been tested and approved (e.g. VAH or FDA approval or CE mark) and which are compatible with the product’s materials according to the chemical manufacturers’ recommendations may be used for processing the product. All the chemical manufacturer's application specifications must be strictly observed. Failure to do so can result in the following problems: ■ Optical changes in materials, e.g., fading or discoloration of titanium or aluminum. For aluminum, the application/process solution only needs to be of pH >8 to cause visible surface changes. ■ Material damage such as corrosion, cracks, fracturing, premature aging, or swelling. ► Do not use metal cleaning brushes or other abrasives that would damage the product surface and could cause corrosion ► For further detailed information on hygienically safe and material-preserving/value-preserving reprocessing, see www.a-k-i.org, link to Publications, Red Brochure – Proper maintenance of instruments.
Disassembling the product before carrying out the reprocessing procedure ► Disassemble the product immediately after use, as described in the respective instructions for use. ► Remove the sealing cap from the Luer lock connector.
D–W: FD–W:
Drinking water Fully desalinated water (demineralized, low microbiological max. 10 microorganisms/ml, low endotoxin: max. 0.25 endotoxin units/ml)
contamination:
Phase I ► Fully immerse the product in the cleaning solution. Ensure that all accessible surfaces are moistened. ► Clean the product immersed in the solution, using a soft cloth or, if necessary, a suitable cleaning brush, until all visible residues have been removed from the surfaces. ► Brush through all surfaces that are not accessible to visual inspection, e.g., in products with hidden crevices, lumens, or complex geometry, for at least 1 minute or until no more residues can be removed. Mobilize non-rigid components, e.g. set screws, joints, slides, etc. 3 times in each direction to the positive stop. ► After cleaning, use a 20-ml disposable syringe to thoroughly rinse these parts of the product that are difficult to access at least 5 times. ► Do not use metal cleaning brushes or other abrasives that would damage the product surface and could cause corrosion Phase II ► Completely rinse/rinse through the product (all accessible surfaces) 3 times for at least 1 min. Mobilize non-rigid components, e.g. set screws, joints, slides, etc. 3 times in each direction to the positive stop. Use fresh water for every rinse. ► Thoroughly (at least 5 times) rinse through surfaces inaccessible to visual inspection, e.g. in products with hidden crevices or lumens (e.g. empty/working channel) or complex geometries, using a single-use syringe (20 ml). ► Drain any remaining water fully. Phase III ► Fully immerse the product in the disinfectant solution. Ensure that all accessible surfaces are moistened. ► Thoroughly (at least 5 times) rinse through surfaces inaccessible to visual inspection, e.g. in products with hidden crevices or lumens (e.g. empty/working channel) or complex geometries, using a single-use syringe (20 ml). Mobilize non-rigid components, e.g. set screws, joints, slides, etc. 3 times in each direction to the positive stop. Phase IV ► Following disinfection, rinse through the product completely (all accessible surfaces) 3 times for at least 2 minutes. Mobilize non-rigid components, e.g. set screws, joints, slides, etc. 3 times in each direction to the positive stop. Use fresh water for every rinse. ► Thoroughly (at least 5 times) rinse through surfaces inaccessible to visual inspection, e.g. in products with hidden crevices or lumens (e.g. empty/working channel) or complex geometries, using a single-use syringe (20 ml). ► Drain any remaining water fully. Phase V ► Dry the product with soft, lint-free tissue. ► Dry inaccessible areas with compressed air (p max= 5 bar).
Mechanical cleaning/disinfecting Preparations at the place of use ► If applicable, rinse surfaces that are not accessible to visible inspection (preferably with demineralized water),
using a disposable syringe, for example. ► Remove any visible surgical residues as much as possible with a damp, lint-free cloth. ► Place the dry product in a sealed waste container and forward it on for cleaning and disinfection within 6 hours.
Preparation before cleaning ► Dismantle the product prior to cleaning, see Disassembling.
Cleaning/disinfection Product-specific safety notes on the reprocessing procedure
Note The cleaning and disinfecting machine must be of tested and approved effectiveness (e.g. FDA approval or CE mark according to DIN EN ISO 15883). Note The cleaning and disinfection device used for processing must be serviced and checked at regular intervals. Note Only use cleaning agents that are suitable for rigid endoscopes. ► Place the instrument in a tray that is suitable for cleaning (avoiding rinsing blind spots). ► Connect components with lumens and channels directly to the rinsing port of the injector carriage. ► Ensure that water can flow out of openings. ► Products with a sheath diameter of ≤4 mm must be cleaned and sterilized in special Aesculap optics trays only. ► Process the product in a cleaning/disinfecting machine. Follow the instructions of the washing machine’s man-
ufacturer.
CAUTION
Damage to the product due to inappropriate cleaning/disinfecting agents and/or excessive temperatures! ► Use cleaning and disinfecting agents according to the manufacturer’s instructions which – are approved for rigid endoscopes, – do not attack softeners (e.g. in silicone). ► Observe specifications regarding concentration, temperature and exposure time. ► Do not exceed the maximum permitted cleaning temperature of 55 °C. Damage to the optical system caused by loosening of connections during ultrasound cleaning! ► Do not clean the endoscope with ultrasound.
► Avoid abrupt cooling of the product (e.g. in water). ► After completion of the mechanical cleaning/disinfecting cycle, inspect all surfaces, cavities, lumens, and open-
ings for visible debris. ► Clean manually if necessary.
Mechanical alkaline cleaning and thermal disinfection Phase
Step
T [°C/°F]
t [min]
Water quality
Chemistry/Note
I
Pre-rinse
<25/77
2
D–W
-
II
Clean
55/131
10
D–W
neodisher MediClean forte 0.5 % (5 ml/L) pH >10
CAUTION III
Rinse I
>10/50
1
D–W
-
► Do not use oxidizing chemicals (e.g. H2O2), which could cause bleaching/layer loss of the product. ► Use suitable cleaning/disinfecting agents if the product is put away in a wet condition. To prevent foam forma-
IV
Rinse II
>10/50
1
FD-W
-
tion and reduced effectiveness of the process chemicals: Prior to mechanical cleaning and disinfection, rinse the product thoroughly with running water.
V
Thermal disinfection
90/194
5
FD–W
-
VI
Dry
-
-
-
According to disinfector program
Manual cleaning/disinfection ► Prior to manual disinfecting, allow water to drip off for a sufficient length of time to prevent dilution of the dis-
infecting solution. ► After manual cleaning/disinfection, check visible surfaces visually for residues. ► Repeat the cleaning /disinfection process if necessary.
D–W: FD–W:
Drinking water Fully desalinated water (demineralized, low microbiological max. 10 microorganisms/ml, low endotoxin: max. 0.25 endotoxin units/ml)
► Check visible surfaces for residues after mechanical cleaning/disinfecting.
contamination:
Technical Service
Inspection, maintenance and checks
CAUTION
Damage (metal seizure/friction corrosion) to the product caused by insufficient lubrication! ► Prior to function checks, lubricate moving parts (e.g. joints, pusher components and threaded rods) at the marked lubrication points, using maintenance oil suitable for the respective sterilization process (e.g. for steam sterilization: Aesculap STERILIT® I oil spray JG600 or STERILIT® I drip lubricator JG598).
Risk of injury and/or malfunction! ► Do not modify the product. WARNING ► For service and repairs, please contact your national B. Braun/Aesculap agency.
► Allow the product to cool down to room temperature. ► After each complete cleaning, disinfecting and drying cycle, check that the instrument is dry, clean, operational,
and free of damage (e.g. broken insulation or corroded, loose, bent, broken, cracked, worn, or fractured components). ► Dry the product if it is wet or damp. ► Repeat cleaning and disinfection of products that still show impurities or contamination. ► Check that the product functions correctly. ► Immediately put aside damaged or inoperative products and send them to Aesculap Technical Service, see Technical Service. ► Assemble dismountable products, see Assembling. ► Check for compatibility with associated products. ► To remove residues of cleaning/disinfecting agents, always wipe distal window 1, illumination window 4 of the light cable connector, and eyepiece glass 6 with a swap moistened with alcohol. ► For products with locking mechanism (e.g. MINOP): Check the locking mechanism for smooth movement.
Packaging ► Appropriately protect products with fine working tips. ► Place the product in its holder or on a suitable tray. Ensure that all cutting edges are protected. ► Pack trays appropriately for the intended sterilization process (e.g. in sterile Aesculap containers). ► Ensure that the packaging provides sufficient protection against recontamination of the product during storage.
Modifications carried out on medical technical equipment may result in loss of guarantee/warranty rights and forfeiture of applicable licenses. ► Prior to dispatching the product for repairs: – Have the product cleaned and disinfected or sterilized, and mark it as “disinfected” or “sterilized”, respectively. – Pack the endoscope in such a way that it will be protected against transport damage. Service addresses Aesculap Technischer Service Am Aesculap-Platz 78532 Tuttlingen / Germany Phone: +49 7461 95-1601 Fax: +49 7461 14-939 E-Mail: [email protected] Other service addresses can be obtained from the address indicated above.
Accessories/Spare parts Replacement parts for Aesculap endoscopes with working channel and valve: Art. no.
Designation
EJ570P
Silicone flap valve (pack of 20)
EJ446255
Sealing cap
EJ751251
Sealing cap for Luer Lock connector (pack of 20)
PM995200
Cleaning brush for working channel
Sterilization Note Alternating sterilization by different sterilization processes can result in damage to materials and adapters of the product. The endoscope can be sterilized through the following processes (as indicated by the symbols on inscription ring 7): ■ Steam sterilization ■ Sterrad® sterilization process: with Sterrad® sterilizers Sterrad® 50, Sterrad® 100S, or Sterrad® 200 ■ EtO sterilization process
Disposal Damage to the optical system caused by flash sterilization! ► Do not flash sterilize the endoscope. ► Do not expose the endoscope to temperatures above 134 °C. CAUTION ► Check to ensure that the sterilizing agent will come into contact with all external and internal surfaces (e.g. by
opening any valves and faucets). ► Do not autoclave damaged products. ► Protect the product against mechanical impacts.
Steam sterilization Note The product can be sterilized either in disassembled or in assembled condition. ► Only autoclave products carrying the symbol for steam sterilization on the inscription ring 7. ► Validated sterilization process
– Disassemble the product, if applicable – Steam sterilization using fractionated vacuum process – Steam sterilizer according to DIN EN 285 and validated according to DIN EN ISO 17665 – Sterilization using fractionated vacuum process at 134 °C/holding time 5 min ► When sterilizing several instruments at the same time in a steam sterilizer, ensure that the maximum load capacity of the steam sterilizer specified by the manufacturer is not exceeded.
Sterrad® sterilization process Sterrad® 50, Sterrad® 100S, Sterrad® 200 Note The product must be disassembled before applying the Sterrad® sterilization process. Note The Sterrad® sterilization process can cause cosmetic changes to the product. These changes will, however, not affect its functionality. ► Disassemble the product. ► Only sterilize endoscopes carrying the SDS symbol for low-temperature and plasma sterilization on the inscrip-
tion ring 7 by means of the Sterrad® sterilization process Sterrad® 50, Sterrad® 100S, Sterrad® 200. ► Unscrew dismountable parts of the endoscope, see Disassembling. ► Sterilization through the Sterrad® sterilization process Sterrad® 50, Sterrad® 100S, Sterrad® 200 Observe the
following: Follow the Sterrad® system manufacturer's instructions. Use of a biological indicator is recommended to confirm effective sterilization.
Ethylene oxide sterilization (EtO sterilization) Note The product must be disassembled before applying the EtO sterilization process. Procedure
Pressure (bar)
T (°C)
Application
EtO sterilization
1,7
55
60 min, 6 % EtO, 94 % CO2
The ethylene oxide and ethylene chlorohydrin resorption by the endoscope materials was tested by an independent test laboratory (DMB Apparatebau, Wiesbaden, Germany), using the Sterivit process. As a result it was verified that the materials are suitable for the ethylene oxide sterilization described above, with the specified degassing time. ► Disassemble the product. ► Perform gas sterilization with ethylene oxide (Sterivit process). Observe the manufacturer’s specifications. ► Allow the product to degas for at least 10 hours.
Storage ► Store sterile products in germ-proof packaging, protected from dust, in a dry, dark, temperature-controlled area.
► Adhere to national regulations when disposing of or recycling the product, its components and its packaging!
TA-Nr. 011057
2018-01
V6
Änd.-Nr. 58216