BBraun
YASARGIL Aneurysm ‐ Slim Clip Applier Forceps Instructions for Use/Technical Description
6 Pages
Preview
Page 1
Clip Applier with latch
Aesculap®
3
4
Aesculap® Neurosurgery USA Instructions for use/Technical description YASARGIL Aneurysm ‐ Slim Clip Applier Forceps
2
Clip Applier without latch 6
1
3
Fig. B 2
3 4 1
2
5
Fig. A Step 1 – Closing Branches
4
2 2
3
Step 2 – Engaging Latch
Step 1 – Closing Branches
2
2
4
Step 3 – Disengaging Latch
Step 2 – Opening Branches 2
4
Step 4 – Opening Branches
Page 1 of 6
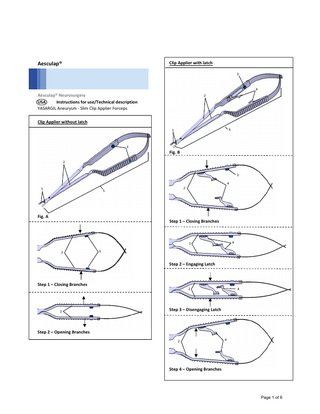
USA Aesculap® YASARGIL Aneurysm – Slim Clip Applier Forceps Legend 1 2 3 4 5
Jaw part Branches left/right Color‐coding button Latches left/right YASARGIL aneurysm clip applier forceps
Symbols on product and packages Caution, general warning symbol Caution, see documentation supplied with the product
to unwanted damage to surrounding tissue resulting in e.g. bleeding, infection, material incompatibilities or instrument parts remaining unnoticed in the patient, etc.
Available sizes All clip applier forceps are marked according to size (Mini or Standard), jaw angulation (Up, Down, Left, Right) and clip material (Phynox or Titanium) so that their correct application with aneurysm clips of the respective size and material is ensured. The clip applier forceps are also available with or without a latch. For detailed information on other available sizes see the YASARGIL aneurysm clips/applier forceps brochure. Spring Laser Marking Identification (Example)
Intended use The Aesculap Slim Clip Applier forceps are used for opening and applying Aesculap YASARGIL aneurysm clips. All clip applier forceps are marked according to size (Mini or Standard) and clip material (Phynox or Titanium) so that their correct application with aneurysm clips of the respective size and material is ensured. Note In the following text, Aesculap YASARGIL aneurysm clips are referred to as aneurysm clips.
Jaw Angulation Laser Marking Identification (Example)
Indications
Safe handling and preparation
The Aesculap Slim Clip Applier forceps are used for opening, closing and applying Aesculap YASARGIL aneurysm clips. Note The manufacturer is not responsible for any use of the product against the specified indications and/or the described applications.
CAUTION Federal law restricts this device to sale by, or on order of a physician! Ensure that the product and its accessories are operated and used only by persons with the requisite training, knowledge, or experience. Read, follow, and keep the instructions for use. Use the product only in accordance with its intended use, see Intended use. Clean the new product mechanically after removing its transport packaging and prior to its initial sterilization. Store any new or unused products in a dry, clean, and safe place. Prior to each use, inspect the product for loose, bent, broken, cracked, worn, or fractured components. Do not use the product if it is damaged or defective. Set aside the product if it is damaged.
Contraindications None known.
MRI Safety Information The clip applier forceps are MR unsafe.
Risks and side effects As part of the legal duty to inform, the following typical risks and side effects associated with the use of surgical instruments are referred to. These are predominantly procedure‐specific, non product‐specific, and not limited
Page 2 of 6
Safe operation Damage to, imprecise functioning and incorrect closing force of the aneurysm clips caused by using the wrong clip applier forceps! Use aneurysm clips only with the matching clip applier forceps; note the respective color of the color‐coding button. Apply long aneurysm clips only with the long clip applier forceps, see TA011547. Observe color of color‐coding button 3. Standard Mini
Titanium aneurysm clips Blue Red
Phynox aneurysm clips Black Gray
Use clips and clip applier forceps only in the following combinations: Phynox aneurysm clips with the clip applier forceps for Phynox aneurysm clips Titanium aneurysm clips with the clip applier forceps for titanium aneurysm clips Mini and Standard aneurysm clips must be applied with the clip applying forceps of the appropriate size (Mini and Standard)
Directions for Use Clip Applier Forceps without latch (Fig. A) Applying Aneurysm Clip Insert the aneurysm clip into jaws 1. Ensure aneurysm clip is full seated in the jaw portion of the clip contact area. Compress the branches left/right 2, see Step 1. The inserted aneurysm clip opens. Fully release branches left/right 2, see Step 2. The aneurysm clip closes. Detach clip applier forceps 5 from the aneurysm clip. Aneurysm clip inserted into the jaw
Ensure aneurysm clip is full seated in the jaw portion of the clip contact area. Compress the branches left/right 2, see Step 1. The inserted aneurysm clip opens. Fully release branches left/right 2, see Step 2. The aneurysm clip closes. Detach clip applier forceps 4 from the aneurysm clip. Note Use caution when handing the clip applier from the surgical nurse to the surgeon. Clip Applier Forceps with latch (Fig. B) Engaging Latch and Retaining Aneurysm Clip Insert the aneurysm clip into jaws 1. Ensure aneurysm clip is full seated in the jaw portion of the clip contact area. Compress the branches left/right 2 (see Step 1) until the left/right latch 4 engage with one another, see Step 2. With the left/right latch 4 engaged, the aneurysm clip is lightly clamped between the jaws 1. Note Use caution when handing the clip applier from the surgical nurse to the surgeon. Disengaging Latch and Applying Aneurysm Clip Compress the branches left/right 2, see Step 3. The inserted aneurysm clip opens. The left/right latch 4 automatically disengage. Fully release branches left/right 2, see Step 4. The aneurysm clip closes. Detach clip applier forceps 6 from the aneurysm clip.
Aneurysm clip fully open Aneurysm clip inserted into the jaw
Aneurysm clip fully open
Re‐Attaching Aneurysm Clip Always take care when re‐attaching the Clip Applier to the aneurysm clip to make sure it is applied at the correct angle. Insert the aneurysm clip into jaws 1.
Page 3 of 6
Validated reprocessing procedure General safety notes Note Adhere to national statutory regulations, national and international standards and directives, and local clinical hygiene instructions for reprocessing. Note For patients with Creutzfeldt‐Jakob disease (CJD), suspected CJD, or possible variants of CJD, observe the relevant national regulations concerning the reprocessing of products. Note It should be noted that successful reprocessing of this medical device can only be guaranteed following prior validation of the reprocessing method. The operator/reprocessing technician is responsible for this. The specified chemistry was used for validation.
manufacturers’ recommendations may be used for processing the product. All the chemical manufacturer’s application specifications must be strictly observed. Failure to do so can result in the following problems: Optical changes in materials, e.g., fading or discoloration of titanium. For aluminum, the application/process solution only needs to be of pH > 8 to cause visible surface changes. Material damage such as corrosion, cracks, fracturing, premature aging, or swelling. Do not use metal cleaning brushes or other abrasives that would damage the product surface and could cause corrosion. For further detailed information on hygienically safe and material‐preserving/value‐preserving reprocessing, see www.a‐k‐i.org, link to Publications, Red Brochure – Proper maintenance of instruments.
Disassembling the product before carrying out the reprocessing procedure Open up products with hinges.
Note For up‐to‐date information about reprocessing and material compatibility, see also the Aesculap USA website at www.aesculapusa.com. The validated steam sterilization procedure was carried out in the Aesculap sterile container system.
General Information Dried or affixed surgical residues can make cleaning more difficult or ineffective and lead to corrosion. Therefore the time interval between application and processing should not exceed 6 h; also, neither fixating pre‐cleaning temperatures >45 °C nor fixating disinfecting agents (active ingredient: aldehydes/alcohols) should be used. Excessive neutralizing agents or basic cleaners may result in a chemical attack and/or fading and the laser marking becoming unreadable either visually or by machine. Residues containing chlorine or chlorides, e.g., in surgical residues, medicines, saline solutions, and in the service water used for cleaning, disinfection, and sterilization, will cause corrosion damage (pitting, stress corrosion) and result in damage to metallic products. These must be removed by rinsing thoroughly with demineralized water and then drying. Perform additional drying, if necessary. Only process chemicals that have been tested and approved (e.g. FDA cleared) which are compatible with the product’s materials according to the chemical
Preparations at the place of use If applicable, rinse surfaces that are not accessible to visible inspection (preferably with demineralized water), using a disposable 10 mL syringe, for example. Remove any visible surgical residues as much as possible with a damp, lint‐free cloth. Place the dry product in a sealed waste container and forward it on for cleaning and disinfection within 6 hours.
Cleaning/disinfection Product‐specific safety notes on the reprocessing procedure Danger to the patient! Reprocess the product only with manual pre‐ cleaning followed by mechanical cleaning. Risk to patient due to cross contamination! Do not clean contaminated products together with uncontaminated products in a tray.
Page 4 of 6
Damage to the product due to inappropriate cleaning/disinfecting agents and/or excessive temperatures! Use cleaning and disinfecting agents according to the manufacturer’s instructions which: o are approved for plastic materials. o do not attack softeners (e.g. in silicone). Observe specifications regarding concentration, temperature and exposure time. Do not exceed the maximum permitted cleaning temperature of 55 °C. Use suitable cleaning/disinfecting agents if the product is put away in a wet condition. Prior to mechanical cleaning and disinfection, rinse the product thoroughly with running water To prevent foam formation and reduced effectiveness of the process chemicals. Clean and disinfect microsurgical products mechanically if they can be placed securely in the machine or on the positioning aids.
Mechanical cleaning/disinfection with manual pre‐ cleaning Note The cleaning and disinfecting machine must be of tested and approved effectiveness (e.g. FDA cleared). Note The cleaning and disinfection machine used for processing must be serviced and checked at regular intervals. Manual pre‐cleaning with a brush Phase
Step
T [°C/°F]
Disinfectant cleaning
RT (cold)
t [min] >15
Conc. [%] 1
Water quality D‐W
Chemicals
I
II
Rinsing
RT (cold)
1
‐
D‐W
‐
D‐W: RT:
Steris Prolystica 2x Concentrate Enzymatic Cleaner
If applicable, brush non‐visible surfaces for at least 1 minute with a suitable cleaning brush. Mobilize non‐rigid components, such as set screws and hinges, during cleaning. Flush these areas thoroughly at least five times with the cleaning disinfectant solution using a disposable syringe (10 ml). Phase II Rinse/flush the instrument thoroughly (all accessible surfaces) under running water. Mobilize non‐rigid components, such as set screws and hinges, during rinsing. Mechanical Cleaning Treatment
Time [mm:ss]
T [°C/°F]
Cleaning Solution
Wash Phase I
05:00
55°C
Steris Prolystica 2x Concentrate Enzymatic Cleaner
Wash Phase II
03:00
Hot Tap Water
Steris Prolystica 2x Concentrate Neutral Detergent*
Rinse
02:00
90°C
Use deionized or distilled water for final rinse
Dry
10:00
90°C
N/A
* Always follow the manufacturer’s specifications for automatic washer‐sterilizers and use a free‐rinsing, low sudsing detergent with a neutral ph (6.0‐8.5). Due to variations in water quality, the type of detergent and its concentrations may require adjustment for optimal disinfection and cleaning. Place the product in a tray that is suitable for cleaning (avoiding rinsing blind spots). Place instruments in the tray with their hinges open. Check visible surfaces for residues after mechanical cleaning/disinfecting.
Drinking water Room temperature
Phase I Fully immerse the product in the cleaning/disinfecting solution for at least 15 minutes. Ensure all accessible surfaces are moistened. Clean the product with a suitable cleaning brush in the solution until all discernible residues have been removed. Page 5 of 6
Sterilization Aesculap advises against sterilizing the device by flash sterilization or chemical sterilization. Sterilization may be accomplished by a standard prevacuum cycle in a steam autoclave. To achieve a sterility assurance level of 10‐6, Aesculap recommends the following parameters: Aesculap Tray/Sterile container (perforated bottom) Minimum cycle parameters* Sterilization method Temp. Time Minimum drying time Prevacuum 132 °C 4 min 20 min
* Aesculap has validated the above sterilization cycle and has the data on file. The validation was accomplished in an Aesculap Sterile container cleared by FDA for the sterilization and storage of instruments. Other sterilization cycles may also be suitable; however individuals or hospitals not using the recommended method are advised to validate any alternative method using appropriate laboratory techniques.
Inspection, maintenance and checks Damage (metal seizure/friction corrosion) to the product caused by insufficient lubrication! Prior to function checks, lubricate moving parts (e.g. joints, pusher components and threaded rods) with maintenance oil suitable for the respective sterilization process. Allow the product to cool down to room temperature. After each complete cleaning, disinfecting and drying cycle, check that the product is dry, clean, operational, and free of damage (e.g. broken insulation or corroded, loose, bent, broken, cracked, worn, or fractured components). Dry the product if it is wet or damp. Repeat cleaning and disinfection of products that still show impurities or contamination. Check that the product functions correctly. Immediately put aside damaged or inoperative products and send them to Aesculap Technical Service, see Technical Service. Check for compatibility with associated products.
Storage Store sterile products in sterile barrier packaging, protected from dust, in a dry, dark, room temperature‐ controlled area per hospital policy.
Technical Service Risk of injury and/or malfunction! Do not modify the product. For service and repairs, please contact your Aesculap representative or customer service. Modifications carried out on medical technical equipment may result in loss of guarantee/warranty rights and forfeiture of applicable licenses. Service address Attn. Aesculap Technical Services 615 Lambert Pointe Drive Hazelwood MO, 63042 Aesculap Repair Hotline Phone: +1 (800) 214‐3392 Fax: +1 (314) 895‐4420 Other service addresses can be obtained from the address indicated above. Instruments returned to Aesculap for repair must have a statement which testifies that each instrument has been thoroughly cleaned and disinfected. Failure to supply evidence of cleaning and disinfection will result in a cleaning charge and delayed processing of your instrument repair. Products repaired by Aesculap are guaranteed for 90 days to be free of defects in the workmanship and parts when used normally for its intended surgical purpose. Any workmanship or parts proving to be defective will be replaced or repaired, at Aesculap’s discretion, at no charge to the customer.
Disposal Adhere to national regulations when disposing of or recycling the product, its components and its packaging!
Contact Information Manufactured for: Aesculap Inc. 3773 Corporate Parkway Center Valley, PA 18034 1(800) 258‐1946 www.aesculapusa.com SOP‐AIC‐5001711 Rev. 4 07/18
Page 6 of 6