BBraun
Yasargil Temporary Aneurysm Clip System
8 Pages
Preview
Page 1
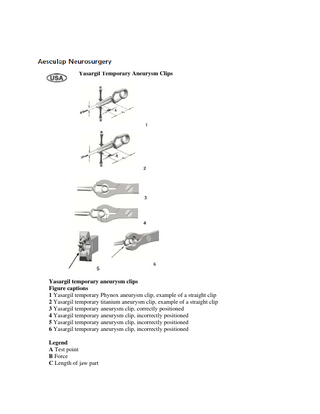
Yasargil Temporary Aneurysm Clips
Yasargil temporary aneurysm clips Figure captions 1 Yasargil temporary Phynox aneurysm clip, example of a straight clip 2 Yasargil temporary titanium aneurysm clip, example of a straight clip 3 Yasargil temporary aneurysm clip, correctly positioned 4 Yasargil temporary aneurysm clip, incorrectly positioned 5 Yasargil temporary aneurysm clip, incorrectly positioned 6 Yasargil temporary aneurysm clip, incorrectly positioned Legend A Test point B Force C Length of jaw part
Symbols on product and packages Sterilization using radiation Use by
Attention, see instructions for use
INDICATIONS FOR USE The Yasargil Aesculap Aneurysm Clips are intended for occlusion of cerebral aneurysms in a temporary manner. They are applied with Aesculap clip appliers, which contain titanium alloy or phynox jaws. “Aesculap Yasargil temporary aneurysm clips” will be referred to as “temporary clips” in the following text. Temporary clips The temporary clips are intended for temporary stasis of cerebral aneurysms. Temporary clips must not be implanted permanently under any circumstances. The temporary clips are intended for multiple uses and can be reused repeatedly. CONTRAINDICATION The temporary clips are contraindicated for all applications except for the temporary stasis of cerebral aneurysms or blood vessels. Temporary clips must not be used for permanent stasis or implantation. PRODUCT DESCRIPTION The temporary clips are available in two different materials: • Cobalt alloy (Phynox) ISO 5832-7 and • Titanium alloy Ti6Al4V, ISO 5832-3. Different models of temporary clips are available. Please contact your local Aesculap sales representative for more information or consult the Yasargil Aneurysm Clip Brochure located at www.aesculapusa.com. The temporary clips are available in three sizes (Mini, Standard and Long).The closing force of each temporary clip is measured individually and printed on the individual packaging. The closing force given on the packaging was measured at 1/3 along the length (from the tip) of the jaw part, at the center of the contact area. The temporary Phynox clips are measured with the jaws opened to 0.5 mm (see Fig. 1) whereas the temporary titanium clips are measured with the jaws opened to 1 mm (see Fig. 2). Each temporary clip carries an individual serial number. The temporary clips are color-coded to differentiate the various sizes and applications.
Special product description for temporary aneurysm clips
Temporary clips are indicated for temporary occlusion of vessels and, therefore, repeated or multiple applications during the same procedure will not adversely affect the efficacy of the aneurysm clip. Safe handling and preparation CAUTION Federal law restricts this device to sale by or on order of a physician! Read and observe the instructions for use, and keep them in a safe place. The product may only be used for its intended application, see Indications for Use. Prior to each use, inspect the product for: loose, bent, broken, cracked, worn, or fractured components. Do not use the product if it is damaged or defective. Products found to be damaged must be set aside immediately. Each temporary clip is individually packaged and sterilized by radiation (min. dose 25 kGy). If the sterile wrapping is open, torn, perforated or otherwise damaged, or when the sterility of the temporary clip has “expired” (use-by date), the aneurysm clip must be regarded as unsterile and has to be sterilized according to the instructions given in the sections on cleaning and sterilizing. Each individually packaged temporary clip is supplied in double sterile packaging including instructions for use and labels. To avoid damage, product malfunction or to avoid any risk of a decrease in closing force: Apply each temporary clip with the appropriate applier and removal forceps. Never use the temporary clips with applier or removal forceps from other manufacturers. The temporary Phynox clips must be applied with the applier for Phynox aneurysm clips. The temporary titanium clips must be applied with the applier and removal forceps for titanium clips. Mini, Standard or Long temporary clips may be used only with the applier and removal forceps of the appropriate size (Mini, Standard or Long). All applier and removal forceps are marked according to size (Mini, Standard or Long) and clip material so that their correct application with clips of the respective size and material is ensured. Additionally, the applier and removal forceps for titanium aneurysm clips can also be color-coded according to clip size. For further information on the suitable applier and removal forceps, please contact your local Aesculap sales representative or consult the Yasargil Aneurysm Clip Brochure which can be located at www.aesculapusa.com.
Carefully pick up the whole temporary clip with the jaw piece of the appropriate applier forceps (see Fig. 3). The temporary clip can be damaged, jump out or slip if picked up improperly (see Fig. 4/5/6). To avoid possible risks to the patient: Temporary clips must not be implanted permanently under any circumstances Aesculap cannot accept any responsibility for temporary clips that are handled inappropriately or not according to the present instructions for use. Safe handling Temporary clips should only be applied by surgeons appropriately trained for, and experienced in, the use of aneurysm clips. If a temporary clip appears changed or shows signs of damage, e.g. incorrect jaw position, bent parts or changed closing force: Set aside the temporary clip.
To avoid damage to the temporary clips: Always handle the temporary clips with appropriate care. Never open a temporary clip with your fingers. Avoid manual and/or mechanical manipulation of the temporary clip. Excessive, rough or repeated handling, especially opening and closing of temporary clips, be it in general use or during cleaning and sterilization, can change the closing force and impair the clinical effectiveness of the temporary clips. As soon as the sterile temporary clip has been brought to the operation field in the usual manner, pick up the temporary clip from its double sterile packaging and position it carefully between the jaws of the applier forceps. The correct positioning of the temporary clip between the jaws of the appropriate applier forceps (see Fig. 3) is crucial here. If the temporary clip is not placed precisely between the jaws parts of the appropriate applier forceps (see Fig. 4/5/6), this can lead to damage to the temporary clip. It could cause a change in the factory-set closing force or result in the temporary clip slipping from the applicator, which could be dangerous during an operation. These directions for use must also be observed when using the removal forceps. The application of temporary clips involves the following severe risks: Shifting or breakage of the aneurysm clip. Yawing of the jaw parts. Rupture of the aneurysm due to punctual, incomplete contact of the clip jaws on the aneurysm neck. For large aneurysms, reduction of the blood vessel cross-section close to the edge of the blood vessel. Cerebrovascular spasms and sudden death. Infections of the operation wound and general surgical complications are other unwelcome (side) effects.
Care and handling Each temporary aneurysm clip in its unopened original packaging has been packed and sterilized individually and is supplied as a sterile product. Unsterile temporary aneurysm clips must be sterilized following the cleaning and sterilization processes outlined in the following section. To preclude damage, inaccurate functioning and an incorrect closing force: Use each temporary clip with the appropriate applier and removal forceps. Never use the temporary clips with applier or removal forceps from other manufacturers. Excessive, rough or repeated handling, especially opening and closing of temporary clips, be it in general use or during cleaning and sterilization, can damage the temporary clip, change the closing force and impair the clinical effectiveness of the temporary clips. To avoid damage to the temporary clips: Always handle the temporary clips with appropriate care. Never open an temporary clip with your fingers. Avoid manual and/or mechanical manipulation of the temporary clip. Special cleaning instructions for temporary aneurysm clips Note Since no reprocessing methods have been validated for removal of transmissible spongiform encephalopathy (TSE) agents from medical devices, this device should not be used for patients with known or suspected TSE agent disease, including CJD and vCJD.
Sterilization must be preceded by the following procedure:
Manual pre-cleaning with ultrasound
Step
Phase
T [°C/°F]
t [min]
Conz. [min]
I
Ultrasonic cleaning
RT (cold)
>15
II
Rinsing
RT (cold)
1
2
WaterQuality
Chemical
D–W
Aldehyde-free, phenoi-free, and QUAT-free concentrate, pH ~ 9
D-W
D-W: Drinking water RT: Room Temperature Phase I Clean the product in an ultrasonic cleaning bath (frequency 35 kHz) for at least 15 min. Ensure that the accessible surfaces are immersed and acoustic shadows are avoided. Phase II Rinse/flush the product thoroughly (all accessible surfaces) under running water.
Mechanical alkaline cleaning and thermal disinfecting
Machine type: single-chamber cleaning/disinfection device without ultrasound Step
T [°C/°F]
t [min]
WaterQuality
I
Prerinse
< 25/77
3
D–W
-
II
Cleaning
55/131
10
FD–W
Concentrate, alkaline: – pH = 13 – <5 % anionic surfactant 0.5 % working solution – pH = 11
III
Intermediate rinse
>10/50
1
FD–W
-
IV
Thermal disinfecting
90/194
5
FD–W
-
V
Drying
Phase
-
Chemcal/Remarks
According to the program for cleaning and disinfection device
D–W: Drinking water FD–W: Fully desalinated water (demineralized, low microbiological contamination: drinking water quality at least) Check visible surfaces for residues after mechanical cleaning/disinfecting.
Inspection Inspect each individual temporary clip. Any temporary clip found with one of the following characteristics must be set aside and excluded from further use: Signs of damage Incorrect jaw position Bent components Changed closing force Misalignment Soilage that cannot be removed The color coding may fade in the course of processing. Temporary clips whose color coding cannot be identified unambiguously anymore must be set aside and excluded from further use. Sterilization method and parameters Each temporary clip in its unopened original packaging has been packed and sterilized individually and is supplied as a sterile product. A temporary clip must be regarded as unsterile e.g. if the sterile packaging is open, torn, perforated or damaged, or if the sterility of the product has “expired” (use-by date). Sterilization has to be carried out through a validated sterilization process. Using a non-validated sterilization process may result in the aneurysm clip being unsterile. Sterilize the temporary clip in the appropriate storage devices recommended by Aesculap for Aesculap Yasargil temporary clips. For further information on the appropriate storage devices recommended by Aesculap, please contact your local Aesculap sales representative or consult the Yasargil Aneurysm Clip Brochure which can be locatd at www.aesculapusa.com.
The temporary clips are supplied sterile. Sterilization of a clip that has been removed from its sterile package may be accomplished by the following the sterilization process outlined in this section. Sterilization of the device may be accomplished by steam. Aesculap does not recommend the device be sterilized by "Flash" or chemical sterilization. Multiple clips may be sterilized together in the same Aesculap rigid sterilization container. Surgical instruments may also be placed within the Aesculap rigid sterilization container (sterile container) for processing under generally accepted hospital in-use conditions.
The recommended sterilization parameters are as follows: Aesculap advises against sterilizing the device by flash sterilization or chemical sterilization. Sterilization may be accomplished by a standard prevacuum cycle in a steam autoclave. To achieve a sterility assurance level of 10-6, Aesculap recommends the following parameters: Aesculap Orga Tray/Sterile container (perforated bottom) Minimum cyle parameters* Sterilization method Temperature Time Prevacuum 270°F 4 min
Min. drying time 20 min
*Aesculap has validated the above sterilization cycle and has the data on file. The validation was accomplished in an Aesculap sterile container cleared by FDA for the sterilization and storage of these products. Other sterilization cycles may also be suitable, however individuals or hospitals not using the recommended method are advised to validate any alternative method using appropriate laboratory techniques. Use an FDA cleared accessory to maintain sterility after processing, such as a wrap, pouch, etc. WARNING for the US market If this device is/was used in a patient with, or suspected of having Creutzfeldt-Jakob Disease (CJD), the device cannot be reused and must be destroyed due to the inability to reprocess or sterilize to eliminate the risk of cross contamination. Storage Store sterile temporary clips in germ-proof packaging, protected from dust, in a dry, dark, temperature-controlled area. For further information on the appropriate storage devices recommended by Aesculap, please contact Aesculap or consult the special brochures for titanium and Phynox temporary clips. These brochures can be ordered from you Aesculap sales representative. Disposal Adhere to national regulations when disposing of or recycling the product, its components and its packaging! Distributor in the US/Contact in Canada for product information and complaints Aesculap, Inc. 3773 Corporate Parkway Center Valley, PA 18034 USA SOP-AIC-5001361 Ver. 01 09/14