Boston Scientific
RF 3000 Product In Service Sept 2014
Product In Service
20 Pages
Preview
Page 1
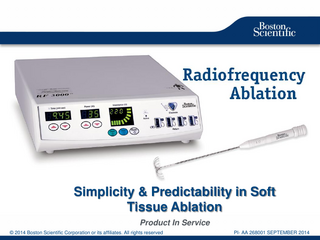
Simplicity & Predictability in Soft Tissue Ablation Product In Service Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 1 2014
PI- AA 268001 SEPTEMBER 2014
Contents
• Product Overview
• Device Preparation • RF3000 Generator: Operation • Product Offering : Matrix Overview
Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 2 2014
PI- AA 268001 SEPTEMBER 2014
Product Overview RFA Soft Tissue Ablation
Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 3 2014
PI- AA 268001 SEPTEMBER 2014
Simple & Predictable thermal ablation
LeVeen electrode are to designed to provide Spherical ablation and Good fixation into the tissue Self-balancing tines with 1-cm spacing enable optimal energy conduction and creation of an progressive and homogenous coagulative necrosis
Development
Progression
Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 4 2014
Expansion
Completion
PI- AA 268001 SEPTEMBER 2014
Broad LeVeenTM Probes Portfolio Array Diameters 2.0-5.0cm
Shaft Lengths 12, 15, 25cm
Printed cm Length Markings
LeVeen™ SuperSlim™ Electrode 17G* shaft**
*17G=1,149mm
** SuperSlim Shaft and handle profile designed to improve puncturability and decrease movements during procedure Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 5 2014
PI- AA 268001 SEPTEMBER 2014
Broad LeVeenTM Probes Portfolio LeVeen™ CoAccess™ Needle Electrode
Soloist™ Needle Electrode
LeVeen CoAccess Needle must be used with CoAccess Introducer
• • •
Pre-Procedural mapping ablation zone of large or complex lesions Cannula may be used as procedural and biopsy access port Fit into CT Gantry
Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 6 2014
Designed for improved puncturability • Small diameter ablation • Lesions close to vital structures PI- AA 268001 SEPTEMBER 2014
Product Overview: Generator
RF 3000™ Generator • Constant Voltage • Frequency: 460 kHz (no muscular contraction) • Power set up (Max 200 watts) • Visual & audible monitoring of Impedance (Roll-Off) • Time display
Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 7 2014
PI- AA 268001 SEPTEMBER 2014
Impedance As a Procedural Endpoint • Application of electrical current results in tissue heating above 50⁰C • Cellular destruction impacts tissue’s ability to conduct current => tissue’s resistance to conduct electrical current = Impedance • A rise in impedance can be used to indicate the completion of a thermal ablation
The RF3000™ Generator Monitors and Displays Impedance Levels Real-time
Measurement of total target tissue destruction Constant Voltage System
™
Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 8 2014
Displays Illuminates Impedance Levels Throughout The Procedure
PI- AA 268001 SEPTEMBER 2014
Device Preparation RFA Soft Tissue Ablation
Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 9 2014
PI- AA 268001 SEPTEMBER 2014
Grounding Pads Placement
RF 3000 is a monopolar system, therefore patients must be grounded Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © 2014 Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 10
PI- AA 268001 SEPTEMBER 2014
Return Pads: Selection & Placement Pad Placement Proper Orientation & Pad Alignment Place the return pads on each leg and align them parallel on the lateral and median part of the thights. The return pads must not ovelap, nor be in contact. Check that they duction properly.
There is a potential for superheating if the return electrodes are placed over an implanted metal prostheses. Return electrodes should not be placed over superficial metal implants. Improper Orientation & Misalignment
Boston Scientific recommends the use of Megadyne™ 0850C Disposable Electrosurgical Patient Return Electrodes (Pads) Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © 2014 Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 11
PI- AA 268001 SEPTEMBER 2014
Pacemakers and ICD’s
For patients with permanent pacemaker and Implantable Cardiac Defibrillators (ICD) additional precautions should be taken. These precautions include, but are not limited to: o Checking with manufacturer of the pacemaker and the patient’s cardiologist regarding functioning of the pacemaker during Radiofrequency Ablation (RFA). o Ensuring that the current path from the RFA site to the disposable patient return electrodes does not pass through the vicinity of the patient’s heart, the implanted pacemaker or the Implantable Cardiac Defibrillator (ICD). o Keeping all RFA cords and cables away from patient’s pacemaker and leads. o Continuous evaluation of the patient’s cardiac rhythm and pacemaker function. o Having a magnet available and a pacemaker programmer present. o Having an external pacemaker available and ready to be activated in the event of prolonged inhibition of the permanent pacemaker.
Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © 2014 Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 12
PI- AA 268001 SEPTEMBER 2014
RF3000™ Generator Operation RFA Soft Tissue Ablation
Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © 2014 Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 13
PI- AA 268001 SEPTEMBER 2014
Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © 2014 Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 14
P4
P3
P2
P1
RF 3000 Generator overview
PI- AA 268001 SEPTEMBER 2014
RF 3000 Generator overview
Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © 2014 Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 15
PI- AA 268001 SEPTEMBER 2014
Generator: Operation
Prior to use, please see the complete “Directions for Use” for more information on Indications, Contraindications, Warnings, Precautions, Adverse Events, and Operator’s Instructions.
Time Display – In Ready mode, displays the time duration for which RF energy will be applied, as entered by the operator. In Operating mode, displays the total elapsed time. Default time is 15:00.
Power Display – During Operating mode, displays the power output. The unit operates as a constant voltage source during Operating mode, so output power will vary as load impedance changes. During Ready mode, this display is blank. Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © 2014 Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 16
PI- AA 268001 SEPTEMBER 2014
Generator: Operation Prior to use, please see the complete “Directions for Use” for more information on Indications, Contraindications, Warnings, Precautions, Adverse Events, and Operator’s Instructions.
Impedance Display – In Operating mode, this display shows both the currently measured impedance and a 10element display of the value relative to the initially measured impedance. During Ready mode, this display is blank.
Active Indicator– Illuminates during Operating mode indicating active RF output (2W or more of RF power). When this indicator is on, measured power and elapsed time are also displayed
Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © 2014 Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 17
PI- AA 268001 SEPTEMBER 2014
Product Offering RFA Soft Tissue Ablation
Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © 2014 Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 18
PI- AA 268001 SEPTEMBER 2014
LeVeen
LeVeen
CoAccess™ SuperSlim™ Electrode Electrode
LeVeen™ Needle Electrodes
Product Offering: Matrix Overview
Soloist™
UPN
Order Num.
Array Dia. (cm)
Cannula Length (cm)
M001262160
26-216
5.0
15
M001262170
26-217
5.0
25
M001262130 M001262310
26-213 26-231
4.0 4.0
15 25
M001262020 M001262030
26-202 26-303
3.5 3.5
12 15
M001262150
26-215
3.5
25
M001262040
26-204
3.0
12
M001262050
26-205
3.0
15
M001262290
26-229
3.0
25
M001262280
26-228
3.0
15
M001262270
26-227
2.0
25
M001262260 M001262220
26-226 26-222
2.0 3.0
15 15
M001262230 M001262240
26-223 26-224
3.5 4.0
15 15
M001262250 M001262500 M001262200
26-225 26-250 26-220
CoAccess Introducer 0.9 18 Generator
Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © 2014 Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 19
LeVeen Needle Electrode
LeVeen CoAccess™ Electrode System
RF 3000 Generator
PI- AA 268001 SEPTEMBER 2014
All cited trademarks are the property of their respective owners. CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. Information for the use only in countries with applicable health authority product registrations." Boston Scientific ConfidentialCorporation -- For Internal Use Do Not Copy, Distribute Externally © 2014 Boston Scientific or Only. its affiliates. AllDisplay rightsorreserved 20
PI- AA 268001 SEPTEMBER 2014