User's Manual
147 Pages
Preview
Page 1
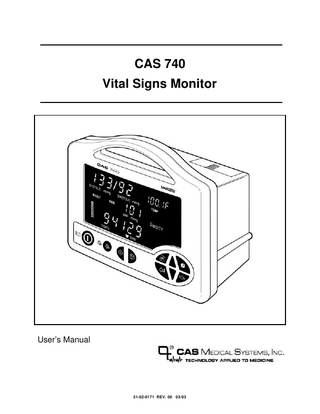
CAS 740 Vital Signs Monitor
User’s Manual
21-02-0171 REV. 00 03/03
21-02-0171 REV. 00 03/03
THE CAS 740 VITAL SIGNS MONITOR
FEATURES CAS 740 - 1
Non-Invasive Blood Pressure and Pulse Rate.
CAS 740 - 2
Non-Invasive Blood Pressure, Pulse Rate and Pulse Oximeter. or Non-Invasive Blood Pressure, Pulse Rate and Temperature.
CAS 740 - 3
Vital Signs Monitor with Non-Invasive Blood Pressure, Pulse Rate, Pulse Oximeter and Predictive Temperature.
IMPORTANT: This manual addresses all parameters of the CAS 740 Vital Signs Monitor. You may have purchased a model that does not have all the parameters referred to in the manual. THIS MANUAL REMAINS SUITABLE FOR USE! Please refer to sections of the manual and the Quick Reference Guide that are applicable for the model purchased.
21-02-0171 REV. 00 03/03
In the U.S. the following Caution applies:
CAUTION: Federal law restricts this device to sale by or on the order of a physician or properly licensed practitioner.
21-02-0171 REV. 00 03/03
Manufacturers Declaration of Conformity Electronic Emissions and Immunity The CAS 740 Monitor is intended for use in the electromagnetic environment specified below. The customer or the user of the CAS 740 Monitor should assure it is used in such an environment. Emissions Test Compliance Electromagnetic Environment RF emissions – CISPR 11 Group 1 The CAS 740 uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. RF emissions – CISPR 11 Class B The CAS 740 is suitable for use in all establishments, Harmonic emissions including domestic establishments and those directly Class B IEC 61000-3-2 connected to the public low-voltage power supply network Voltage fluctuations / flicker Complies that supplies buildings used for domestic purposes. emissions Immunity Test Electrostatic discharge (ESD) IEC 61000-4-2 Electrical fast transient/burst IEC 61000-4-4 Surge IEC 61000-4-5
Voltage Dips, short interruptions and voltage variations on power supply input lines IEC 61000-4-11
IEC 60601 Test Level +/-6 kV contact +/-8 kV air
Compliance Level +/-6 kV contact +/-8 kV air
+/-2 kV for power supply lines +/-1 kV for input/output lines +/-1 kV differential mode +/-2 kV common mode < 5% UT (>95% dip in UT) for 0.5 cycle. 40% UT (60% dip in UT) for 5 cycles. 70% UT (30% dip in UT) for 25 cycles. < 5% UT (> 95% dip in UT) for 5 seconds. 3 A/m
+/-2 kV for power supply lines +/-1 kV for input/output lines +/-1 kV differential mode +/-2 kV common mode < 5% UT (>95% dip in UT) for 0.5 cycle. 40% UT (60% dip in UT) for 5 cycles. 70% UT (30% dip in UT) for 25 cycles. < 5% UT (> 95% dip in UT) for 5 seconds.
Electromagnetic Environment Guidance Floors should be wood concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%. Mains power quality should be that of a typical commercial or hospital environment. Mains power quality should be that of a typical commercial or hospital environment. Mains power quality should be that of a typical commercial or hospital environment. If user of the model 740 requires continued operation during power mains interruptions, it is recommended that the model 740 be powered from an uninterruptible power supply or a battery.
Power frequency 3 A/m Power frequency magnetic fields should (50/60 Hz) be at levels characteristic of a typical magnetic field location in a typical commercial or IEC 61000-4-8 hospital environment. NOTE: UT is the A.C. mains voltage prior to application of the test level.
21-02-0171 REV. 00 03/03
WARRANTY POLICY MONITORS (CAS 740) CAS Medical Systems, Inc. warrants the monitor, when new, to be free from defects in material and workmanship and to perform in accordance with manufacturer’s specifications for a period of two (2) years from the date of original purchase from CAS or its authorized distributors or agents except as noted below. The same warranty conditions are made for a period of one (1) year with respect to printers and battery and ninety (90) days on non-disposable accessories and certain components consisting of reusable SpO2 sensors, reusable temperature probes and other accessories provided by CAS as part of the original purchase. CAS warrants blood pressure cuffs and disposable or single-patient-use products for out-of-box failure only. Where the accessory is not a CAS manufactured product, the manufacturers own warranty conditions apply. CAS reserves the right to perform warranty service operations in its own factory, at an authorized repair facility, or at the customers’ site. Our obligation under this warranty is limited to repairing or, at our option, replacing any defective parts or our equipment, without charge, if such defects occur in normal service and with prompt notification. Damage to any part through misuse, neglect, or accident, or by affixing any accessories or attachments ® ® ® ® other than CAS, Masimo , Nellcor , Nonin , and Welch Allyn manufactured accessories or attachments, is not covered by this warranty.
ACCESSORIES, BATTERIES, CUFFS, AND CERTAIN COMPONENTS In all cases, policy applies from date of purchase from CAS or its authorized distributors or agents.
Accessories: Batteries: Cuffs (all): External Printer: Other Accessories:
Ninety (90) Days - Masimo, Nellcor and Nonin Sensors, Welch Allyn Temperature Probes. One (1) Year Out-of-box failure only. One (1) Year Out-of-box failure only.
THERE ARE NO WARRANTIES, WHICH EXTEND BEYOND THOSE EXPRESSLY DESCRIBED IN THIS AGREEMENT AND THE COMPANY MAKES NO WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE.
21-02-0171 REV. 00 03/03
HOW TO CONTACT US
CAS Medical Systems, Inc 44 East Industrial Road Branford, CT 06405 U.S.A.
Representative in European Union: Mossa Consulting GmbH Bollbrügg 22 23570 Lübeck, Germany
Phone: (800) 227-4414 (203) 488-6056
Phone: +49-4502-880-557
Fax: (203) 488-9438
Fax: +49-4502-880-559
E-Mail: custsrv@casmed.com sales@casmed.com techsrv@casmed.com
E-Mail: mossa.rod@t-online.de Should service be required, contact the dealer in the country of purchase.
Web: www.casmed.com
Copyright 2003 CAS Medical Systems, Inc. All rights reserved. No part of this manual may be reproduced without the written permission of CAS Medical Systems, Inc. CAS reserves the right to make changes to this manual and improvements to the product it describes at any time without notice or obligation.
21-02-0171 REV. 00 03/03
CAS 740 Monitors TABLE OF CONTENTS
1
INTRODUCTION AND INTENDED USE ... 1 INTRODUCTION ... 3 INDICATIONS FOR USE ... 3 CONTRAINDICATIONS ... 3 BRIEF DEVICE DESCRIPTION ... 3 PATIENT ENVIRONMENT ... 4
2
UNPACKING THE MONITOR ... 7 INITIAL INSPECTION ... 9 MONITOR CHECKLIST ... 9 OPTIONAL ACCESSORIES ... 9
3
SYMBOLS ... 11
4
SAFETY MEASURES, WARNINGS AND PRECAUTIONS ... 17 AUTOMATIC SAFETY FEATURES ... 22
5
BLOOD PRESSURE MONITORING ... 23 CUFF SELECTION AND APPLICATION ... 25 NIBP HOSES ... 27
6
PULSE OXIMETRY MONITORING ... 29 TAKING AN SpO2 MEASUREMENT ... 31 FINGER CLIP SENSORS ... 31 DISPOSABLE FLEX-TYPE SENSORS ... 32 MASIMO OXIMETRY ... 33 ATTACHING THE CABLES ... 33 REMOVING THE INTERFACE CABLE ... 34 MASIMO MESSAGES ... 34 NELLCOR OXIMETRY ... 35 ATTACHMENT PROCEDURE ... 35 REMOVING THE INTERFACE CABLE ... 35
21-02-0171 REV. 00 03/03
i
CAS 740 Monitors NONIN OXIMETRY ... 36 ATTACHING THE SENSOR CABLE ... 36 REMOVING THE SENSOR CABLE ... 36
7
TEMPERATURE MONITORING ... 37 WELCH ALLYN TEMPERATURE ... 39 TAKING AN ORAL TEMPERATURE ... 39 TAKING A RECTAL TEMPERATURE ... 41 TAKING AN AXILLARY TEMPERATURE ... 43
8
MONITOR OPERATION ... 45 FRONT PANEL ... 47 DIGITAL DISPLAY AND INDICATORS ... 47 FRONT PANEL CONTROLS ... 49 INFRARED DATA PORT ... 51 REAR PANEL ... 52 AC/DC CONNECTOR ... 52 FUSE COMPARTMENT ... 52 BATTERY COMPARTMENT ... 52 LEFT SIDE PANEL ... 53 MAX NIBP CUFF HOSE CONNECTION ... 53 SpO2 SENSOR CONNECTION ... 53 RIGHT SIDE PANEL ... 54 TEMPERATURE PROBE ELECTRICAL CONNECTION ... 54 TEMPERATURE HOLDER ... 54 EXTERNAL DEVICE INTERFACING ... 54 MONITOR OPERATING INSTRUCTIONS ... 55 ADULT/ NEONATE OPERATING MODE ... 55 TURNING THE CAS 740 MONITOR “ON” ... 55 FRONT PANEL INTESITY CONTROL ... 56 DISPLAYING THE TIME ... 56 MANUAL MODE FOR BLOOD PRESSURE DETERMINATION ... 56 AUTOMATIC CYCLE FOR BLOOD PRESSURE DETERMINATION ... 58 STAT MODE ... 58 HISTORY MODES ... 59 EVENT HISTORY ... 60 TREND HISTORY ... 60 PRINT HISTORY ... 61 CLEARING HISTORY ... 61 REAL TIME CLOCK ... 61
21-02-0171 REV. 00 03/03
ii
CAS 740 Monitors PATIENT ALARM MODE ... 62 CHANGING ALARM LIMITS ... 62 SAVING ALARM LIMITS ... 62 RESTORE FACTORY DEFAULTS ... 63 ALARM LIMIT VALUES ... 63 AUDIBLE AND VISUAL INDICATORS ... 64 CLEARING ALARMS ... 65 NIBP PATIENT ALARMS ... 65 HIGH / LOW %SpO2 ALARMS ... 65 SpO2 PULSE RATE ALARM ... 65 EQUIPMENT ALARMS ... 65 ADJUSTING THE AUDIO ALARM VOLUME ... 66 ADJUSTING THE SpO2 “BEEP” VOLUME ... 66 2-MINUTE AUDIO SILENCE ... 66 PERMANENT AUDIO ALARM SILENCE ... 67 ALARM LIMITS OFF ... 67 MONITOR CONFIGURATION ... 68 ENTERING THE MONITOR CONFIGURATION MENU ... 68 SAVING YOUR CHANGES ... 68 SOFTWARE REVISIONS ... 69 SETTING THE LANGUAGE ... 69 SELECTING THE PATIENT MODE ... 69 SELECTING THE TEMPERATURE SCALE ... 70 AUDIO ALARMS (SILENCE/RESET PUSHBUTTON) ... 70 2-MINUTE AUDIO ALARM OFF ... 70 PERMANENT AUDIO ALARM OFF ... 71 ALARM LIMIT OFF ... 71 MAP VALUE ENABLE / DISABLE ... 71 SETTING THE DATE ... 72 SETTING THE TIME ... 72 DAYLIGHT SAVING TIME OPTION ... 72 BATTERY POWER ... 73 CHECKING BATTERY STATUS ... 74 AUTO OFF FEATURE ... 74 POWER FAIL ... 75 USER MESSAGES ... 75 SpO2 USER MESSAGES ... 75 TEMPERATURE FUNCTION USER MESSAGES ... 77 ERROR MESSAGES ON THE MESSAGE WINDOW ... 78
9
EXTERNAL PRINTER ... 81 PRINTER OVERVIEW ... 83 PRINTER CONTROLS AND INDICATORS ... 84 PRINTER OPERATIONS ... 85 CHARGING THE PRINTER BATTERY ... 87 INSTALLING PAPER ... 87 REPLACING THE BATTERY PACK ... 88 INSTALLING A NEW BATTERY PACK ... 89
21-02-0171 REV. 00 03/03
iii
CAS 740 Monitors
14
ACCESSORIES ... 113 BLOOD PRESSURE CUFFS ... 115 INFLATION HOSES ... 115 OXIMETRY ... 116 MASIMO ... 116 NELLCOR ... 116 NONIN ... 116 TEMPERATURE ... 117 WELCH ALLYN ... 117 OTHER ACCESSORIES ... 117
15
GLOSSARY ... 119
16
SPECIFICATIONS ... 123
CAS 740 MONITOR PURCHASING RECORD ... 130
21-02-0171 REV. 00 03/03
v
CAS 740 Monitors FIGURES Figure 1 ... 4 Patient Environment Figure 2 ... 25 Cuff Application Figure 3 ... 26 Cuff Positioning Figure 4 ... 31 SpO2 Finger Clip Sensor Application Figure 5 ... 32 Flex – Type Adult Application Figure 6 ... 32 Flex – Type Infant Application Figure 7 ... 32 Flex – Type Neonatal Application Figure 8 ... 39 Loading the Probe Cover Figure 9 ... 40 Location of Sublingual Pockets Figure 10... 42 Loading the Probe Cover Figure 11... 43 Loading the Probe Cover Figure 12... 47 Front Panel View Figure 13... 49 Front Panel Controls Figure 14... 52 Rear Panel View Figure 15... 53 Left Side Panel View Figure 16... 54 Right Side Panel View
21-02-0171 REV. 00 03/03
vi
CAS 740 Monitors
Figure 17... 84 Printer Controls and Indicators Figure 18... 86 Sample Printouts Figure 19... 87 Paper Installation Figure 20... 88 Opening the Battery Door Figure 21... 89 Installing the New Battery Figure 22... 106 Removing the Monitor Battery Pack Figure 23... 112 DB9 Male Connector Pin Layout
21-02-0171 REV. 00 03/03
vii
CAS 740 Monitors TABLES Table 1... 5 Parts of the System Table 2... 27 Neonatal Cuff Size Selection Table 3... 27 Adult – Infant Cuff Size Selection Table 4... 57 Selectable Initial Inflation Pressures Table 5... 63 Default Alarm Values Table 6... 64 Audible and Visual Indicators Table 7... 69 Software Revisions Table 8... 77 Temperature Error Codes Table 9... 78 Error Messages on the Message Window Table 10... 112 DB9 Pin Out Table 11... 118 Monitor Configurations
21-02-0171 REV. 00 03/03
viii
CAS 740 Monitors
Section
1
Introduction and Intended Use
21-02-0171 REV. 00 03/03
1
CAS 740 Monitors
21-02-0171 REV. 00 03/03
2
CAS 740 Monitors INTRODUCTION AND INTENDED USE INTRODUCTION The CAS 740 Monitor is multi parameter monitor measuring blood pressure, oxygen saturation and temperature. Non-invasive blood pressure is measured using the oscillometric technique determining systolic, diastolic, mean arterial pressure and pulse rate. The pulse oximeter function continuously monitors and displays values for functional arterial hemoglobin saturation and a pulse rate. Temperature is obtained in the normal (predictive) mode in as little as four (4) seconds. A monitoring mode is available for taking axillary temperatures.
INDICATIONS FOR USE The CAS 740 Monitor is a portable device intended to be used by trained clinicians for multi-parameter vital signs monitoring of neonatal, pediatric and adult patients in health care bedside applications as well as for intra-facility or inter-facility and EMS transport. Parameters displayed are the non-invasive blood pressure (systolic, diastolic and mean arterial pressure), pulse rate, functional oxygen saturation of arterial hemoglobin (%SpO2) and temperature.
CONTRAINDICATIONS • • • •
Oral and Rectal Temperature measurements are not intended for neonatal use. Reusable SpO2 sensor is contraindicated for use for prolonged periods of use. It is not intended for long term monitoring. It must be removed and repositioned every four (4) hours and if indicated by circulatory condition or skin integrity, reapplied to a different monitoring site. Disposable SpO2 sensors are contraindicated for patients that exhibit allergic reactions to adhesive tape. The sensors must be removed and repositioned every eight (8) hours and if indicated by circulatory condition or skin integrity, reapplied to a different monitoring site. No other contraindications are known at this time.
BRIEF DEVICE DESCRIPTION The CAS 740 Monitor is compact, lightweight and portable, allowing it to be easily carried and used in a variety of clinical settings. The monitor is powered by AC Line Power, +12 VDC or by a Nickel Metal Hydride (NiMH) rechargeable battery pack. The internal battery pack charges when the monitor is plugged into a power source (AC Line Power or +12 VDC). The CAS 740 Monitor can be set to operate in one of eight (8) different languages: English, German, French, Italian, Spanish, Dutch, Swedish or Portuguese. The message window can display various system alarm messages. These messages direct the user to check conditions such as the battery state, air leaks and measurement problems. The message window also displays the operational mode of the monitor (automatic or manual). The non-invasive blood pressure (NIBP) parameter automatically inflates an occluding cuff and, using the oscillometric measurement technique, determines systolic, diastolic and mean arterial pressure and pulse rate. Measurement results along with operator prompts and error messages are displayed on the front panel. The frequency of NIBP determination can be selected by the operator in varied times between one and ninety minutes. The auto and manual operating modes cover a variety of clinical uses.
21-02-0171 REV. 00 03/03
3
CAS 740 Monitors The pulse oximeter parameter (%SpO2) determines arterial oxyhemoglobin saturation by measuring the absorption of red and infrared light passing through the tissue. Changes in absorption caused by pulsations of blood in the vascular bed are used to determine arterial saturation and pulse rate. The oximeter requires no routine calibration or maintenance. Oxygen saturation and heart rate are displayed on light emitting diode (LED) digital displays. On each detected pulse, the perfusion LED does indicate patient perfusion signals. This bar graph gives the user a pulse-by-pulse visual indication of waveform signal quality. An audio “beep” can be enabled that is generated each time the SpO2 sensor detects a pulse.
NOTE: The bar graph is not proportional to the pulse volume. The temperature parameter has the capability of taking temperature in either normal (predictive) or monitor mode. In the normal mode, the thermometer’s microprocessor “predicts” body temperature in about four (4) seconds for oral temperatures, about ten (10) seconds for axillary temperatures and in about fifteen (15) seconds for rectal temperatures. Monitor mode is normally used for longer term monitoring and when difficult situations prevent accurate temperature from being taken in the predictive mode. In monitoring mode, the probe must be in contact with tissue for at least three (3) minutes for accurate oral / rectal temperature measurement and five (5) minutes for accurate axillary temperature measurement. The default setting used by the CAS 740 Monitor for temperature determinations is the normal (predictive) mode.
NOTE: Axillary temperature readings may only be taken in the Neonate monitoring mode.
PATIENT ENVIRONMENT The CAS 740 Monitor has been tested with specific parts of the “system” used within the Patient Environment. Figure 1, defines the Patient Environment.
FIGURE 1 Patient Environment
21-02-0171 REV. 00 03/03
4
CAS 740 Monitors The parts of the CAS 740 Monitor “system” that can be used in the Patient Environment are defined as;
The CAS 740 Monitor Appropriate Accessories, listed in the ACCESSORIES section of this User’s Manual Line Cord Optional RS232 / Nurse Call Option Citizen CMP-10 Mobile Printer RS232 Interconnect Cable (supplied with printer) AC Adapter / Charger, Model TRC-09-1100-M from Group West (supplied with printer)
TABLE 1 Parts of the System
21-02-0171 REV. 00 03/03
5
CAS 740 Monitors
21-02-0171 REV. 00 03/03
6