User Manual
94 Pages
Preview
Page 1
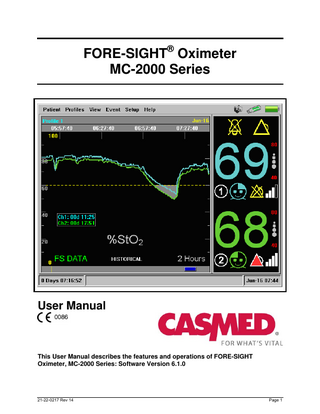
FORE-SIGHT® Oximeter MC-2000 Series
User Manual
This User Manual describes the features and operations of FORE-SIGHT Oximeter, MC-2000 Series: Software Version 6.1.0
21-22-0217 Rev 14
Page 1
FORE-SIGHT Oximeter, MC-2000 Series
Overview Trademarks Trademarked names appear throughout this document. Instead of inserting a trademark symbol with each mention of the trademarked name, the publisher states that it is using the names only for editorial purposes and to the benefit of the trademark owner with no intention of improperly using that trademark. is a registered trademark of CAS Medical Systems, Inc. FORE-SIGHT® is a registered trademark of CAS Medical Systems, Inc. LASER-SIGHT® is a registered trademark of CAS Medical Systems, Inc. COOL-LIGHT® is a registered trademark of CAS Medical Systems, Inc. HOLD-TIGHT® is a registered trademark of CAS Medical Systems, Inc.
Contact Addresses CAS Medical Systems, Inc. 44 East Industrial Road Branford, CT 06405 U.S.A. Phone: in the US: (800) 227-4414 +1 (203) 488-6056
EC
REP
MediMark® Europe 11 rue E. Zola 38100 Grenoble. France
Fax: +1 (203) 488-9438 E-Mail: custsrv@casmed.com sales@casmed.com techsrv@casmed.com Web: www.casmed.com Please contact the distributor in the country of purchase if product information or service should be required.
21-22-0217 Rev 14
Page 2
FORE-SIGHT Oximeter, MC-2000 Series
Manufacturer’s Declaration of Conformity Manufacturers Declaration of Conformity Electronic Emissions and Immunity The FORE-SIGHT Oximeter, MC-2000 Series is intended for use in the electromagnetic environment specified below. The customer or the user of the Oximeter should assure it is used in such an environment. Emissions Test Compliance Electromagnetic Environment RF emissions – CISPR 11 Group 1 The Oximeter uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. RF emissions – CISPR 11 Class A The Oximeter is suitable for use in all establishments other than Harmonic emissions domestic establishments and those directly connected to the public Class A IEC 61000-3-2 low-voltage power supply network that supplies buildings used for Voltage fluctuations / flicker Complies domestic purposes. emissions Immunity Test
IEC 60601 Test Level
Compliance Level
Electrostatic discharge (ESD) IEC 61000-4-2
Level 3
Level 3
Electrical fast transient/burst IEC 61000-4-4
±2 kV for power supply lines ±1 kV for input/output lines
±2 kV for power supply lines ±1 kV for input/output lines
Surge IEC 61000-4-5
±1 kV line(s) to line(s) mode ±2 kV line(s) to earth mode < 5% UT (> 95% dip in UT) for 0.5 cycle. 40% UT (60% dip in UT) for 5 cycles. 70% UT (30% dip in UT) for 25 cycles. < 5% UT (> 95% dip in UT) for 5 s 3 A/m
±0.5 kV line(s) to line(s) mode ±2 kV line(s) to earth mode < 5% UT (> 95% dip in UT) for 0.5 cycle. 40% UT (60% dip in UT) for 5 cycles. 70% UT (30% dip in UT) for 25 cycles. < 5% UT (> 95% dip in UT) for 5 s 3 A/m
Voltage dips, short interruptions and voltage variations on power supply input lines IEC 61000-4-11
Power frequency (50/60 Hz) magnetic field IEC 61000-4-8 NOTE: UT is the A.C. mains voltage prior to application of the test level.
21-22-0217 Rev 14
Electromagnetic Environment Guidance The Oximeter is designed for use in controlled environments only. Per OSHA guidelines for operating rooms, the area must employ adequate static electricity controls. The relative humidity should be maintained at about 50%. The Oximeter is designed for use in controlled environments only. Per OSHA guidelines for operating rooms, the Mains power quality should be that of a typical commercial or hospital environment. Mains power quality should be that of a typical commercial or hospital environment. Mains power quality should be that of a typical commercial or hospital environment. If user of the Oximeter requires continued operation during power mains interruptions, it is recommended that the Oximeter be powered from an uninterruptible power supply or a battery. Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment.
Page 3
FORE-SIGHT Oximeter, MC-2000 Series
Guidance and Manufacturer’s Declaration – Electromagnetic Immunity The FORE-SIGHT Oximeter, MC-2000 Series is intended for use in the electromagnetic environment specified below. The customer or the user of the Oximeter should insure that it is used in such an environment. Immunity Test IEC 60601 Test Level Compliance Electromagnetic Environment – Guidance Level Portable and mobile RF communications equipment should be used no closer to any part of the Oximeter, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter. Recommended separation distance: Conducted RF IEC 61000-4-6
3 Vrms 150 kHz to 80 MHz
3 Vrms
Radiated RF IEC 61000-4-3
3 V/m 80 MHz to 2.5 GHz
3 V/m
d = 1.2√P d = 1.2√P 80 MHz to 800 MHz d = 2.3√P 800 MHz to 2.5 GHz Where P is the maximum output power rating of the transmitter in watts according to the transmitter manufacturer and d is the recommended separation distance in meters. Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey a , should be less than the compliance level in each b frequency range. Interference may occur in the vicinity of equipment marked with the following symbol:
NOTE 1 At 80 MHz and 800MHz, the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is effected by absorption and reflection from structures, objects and people. a Field strengths from fixed transmitters, such as base stations for radio (cellular / cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the Oximeter is used exceeds the applicable RF compliance level above, the Oximeter should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the Oximeter. b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
Recommended Separation Distances Between Portable and Mobile RF Communications Equipment and the Oximeter The Oximeter is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the Oximeter can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the Oximeter as recommended below, according to the maximum output power of the communications equipment. Separation distance according to frequency of transmitter (Meters) Rated maximum output power of transmitter (Watts)
150 kHz to 80 MHz
80 MHz to 800 MHz
d = 1.2√P
d = 1.2√P
800 MHz to 2.5 GHz d = 2.3√P
0.01 0.12 0.12 0.23 0.1 0.38 0.38 0.73 1 1.2 1.2 2.3 10 3.8 3.8 7.3 100 12 12 23 For transmitters operating at a maximum output power not listed above, the recommended separation distance d in meters can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts according to the transmitter manufacturer. NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects, and people.
21-22-0217 Rev 14
Page 4
FORE-SIGHT Oximeter, MC-2000 Series
CE Marking Information Compliance The FORE-SIGHT Oximeter, MC-2000 Series bears the CE mark CE-0086 indicating conformity with the provisions of the Council Directive 93/42/EEC concerning medical devices and fulfills the essential requirements of Annex I of this directive. Exceptions None
Conventions Used in this Manual Warning: Directions that warn of conditions that put the patient or the caregiver at risk. Caution: Directions that help to avoid damaging the Oximeter or losing data. Note: Directions that make it easier to use the Oximeter, something not readily apparent.
21-22-0217 Rev 14
Page 5
FORE-SIGHT Oximeter, MC-2000 Series
General Information This manual is an integral part of the product and describes its intended use. Compliance with the manual is a prerequisite for proper product performance and correct operation and ensures patient and operator safety. Refer to Symbols starting on page 82 for additional information. The warranty does not cover damages resulting from the use of accessories and consumables from other manufacturers. CAS Medical Systems, Inc., (CASMED) is responsible for the effects on safety, reliability, and performance of the product only if: • Assembly, operations, extensions, readjustments, modifications, or repairs are carried out by persons authorized by CASMED. • The electrical installation of the relevant room complies with the requirements of the appropriate regulations. • The device is used in accordance with the instructions for use. • All publications conform to the product specifications and applicable IEC publications on safety and essential performance of electro-medical equipment as well as with applicable UL requirements and AHA recommendations valid at the time of printing. For complete warranty information, refer to the Warranty Policy located on page 87. The CASMED quality management system complies with the international standards ISO 13485 and the Council Directive on Medical Devices 93/42/EEC. Note: Due to continuing product innovation, specifications in this manual are subject to change without notice. Warning: Before using the Oximeter for the first time, please read the information given in section Safety, starting on page 14. Warning: Before using Sensors for the first time, please read the instructions for use provided with the Sensors. In the U.S. the following Caution applies: Caution: Federal law restricts this device to sale by or on the order of a physician or licensed practitioner.
21-22-0217 Rev 14
Page 6
FORE-SIGHT Oximeter, MC-2000 Series
About This Manual This User Manual describes the features and operation of the FORE-SIGHT Oximeter: Software Version 6.1.0 Note: This manual addresses all parameters an Oximeter can have installed. It remains suitable for use if the Oximeter has a sub-set of parameters only. Please refer to those sections that are applicable for the model in use.
Manual Purpose This manual contains the instructions necessary to operate the Oximeter safely and in accordance with its functions and intended use. Note: All illustrations in this manual are provided as examples only. They may not necessarily reflect your monitoring setup or data displayed on your Oximeter.
Intended Audience This manual is written for clinical professionals. Clinical professionals are expected to have working knowledge of medical procedures, practices, and terminology as required for monitoring of critically ill patients. Caution: For continued safe use of this equipment, it is necessary that the listed instructions be followed. However, instructions listed in this manual in no way supersede established medical practices concerning patient care.
21-22-0217 Rev 14
Page 7
FORE-SIGHT Oximeter, MC-2000 Series
Revision History This manual has a revision number located at the bottom of each page. It changes whenever the manual is updated. Rev 00 Rev 01 Rev 02 Rev 03 Rev 04 Rev 05 Rev 06 Rev 07 Rev 09 Rev 09 Rev 10 Rev 11 Rev 12 Rev 13 Rev 14
04/2007 05/2007 05/2007 07/2007 10/2007 12/2007 02/2008 06/2008 03/2009 04/2009 07/2009 08/2009 01/2010 10/2010 02/2011
Read this manual carefully before patient use of the Oximeter CASMED reserves the right to make changes to this manual and improvements to the product it describes at any time without notice or obligation. Copyright 2007-2011 CASMED. All rights reserved.
21-22-0217 Rev 14
Page 8
FORE-SIGHT Oximeter, MC-2000 Series
Contents Overview 2 Trademarks ... 2 Contact Addresses ... 2 Manufacturer’s Declaration of Conformity 3 CE Marking Information... 5 Conventions Used in this Manual ... 5 General Information... 6 About This Manual... 7 Manual Purpose... 7 Intended Audience ... 7 Revision History ... 8 Safety 14 Indications for Use ... 14 Contraindications... 14 Installation and Setup ... 15 Device Handling ... 17 Safety Checks ... 18 Monitoring... 19 Initial Inspection... 21 Oximeter Checklist ... 21 Patient Environment ... 22 Oximeter Classifications of Electrical Insulation ... 22 Basic Operations 23 Introduction... 23 Getting Started ... 23 Physical Configuration... 24 Front View... 24 Rear View ... 25 Oximeter Keys and Controls... 26 Rotary knob... 26 On / Standby Key ... 27 Alarm Silence / Reset Key ... 27 Programmable SoftKey... 27 Sensor Start / Restart Key ... 27 Oximeter Display ... 28 Parts of the Display Screen ... 28 Menu Navigation ... 29 Patient Menu... 29 New Patient Menu... 30 Profiles Menu ... 32 View Menu ... 32 Event Menu... 33 Setup Menu... 34 Help Menu... 34 About Menu... 35 Entering Text... 35
21-22-0217 Rev 14
Page 9
FORE-SIGHT Oximeter, MC-2000 Series
Setting Up the Oximeter for the Patient ... 36 Labeling the Data with the Patient Identifier ... 37 Selecting a User Profile ... 37 Connecting the Preamp Cables... 38 Connecting the Preamp Cables for the First Time ... 38 Removing the Preamp Cables ... 41 Connecting the Sensors ... 42 Connecting the Sensor to Preamp Cables... 42 Entering Patient Information ... 44 Disconnecting the Sensor from Preamp Cable ... 45 Affixing the Sensor to the Patient ... 45 Preparing the Patient ... 46 Applying the Sensors ... 46 TPI Indicator... 47 Removing the Sensors... 47 Starting the Oximeter... 48 Turning the Oximeter On ... 48 Starting to Monitor a Patient ... 48 Configuring Options on the View and Setup Menus ... 49 Selecting a View ... 49 Setting Up Alarm Limits and Volume ... 50 Adjusting a Slider Bar ... 51 Setting User Preferences... 51 Setting Display Brightness ... 52 Setting Auto Dim ... 53 Deactivating Auto Dim Temporarily ... 53 Selecting the Language ... 53 Selecting the Serial Ports... 54 Connecting to Philips IntelliVue ... 55 Serial Port Data Output... 56 Setting the Date and Time ... 57 Monitoring the Patient... 58 Controlling the Alarm ... 58 Enabling the Threshold Analysis... 58 Setting the Analysis Threshold ... 59 Positioning an Event Cursor over the Patient Data... 59 Adding an Event to Menu... 60 Adding a new Event to the Patient Record ... 61 Placing an Event on the Patient Record ... 62 Change Existing Events... 64 Undo the Last Event ... 64 Switching between Active and Historical Mode ... 65 Reviewing Patient History ... 65 Responding to System Messages ... 66 FORE-SIGHT (FS) Data Collections ... 70 Starting FS Data Collection... 70 Stopping FS Data Collection... 70 Reviewing FS Data Information ... 71
21-22-0217 Rev 14
Page 10
FORE-SIGHT Oximeter, MC-2000 Series
USB
72 Memory Sticks ... 72 Saving Patient Data ... 72 USB Status ... 74 Screen Snapshots... 75
Cleaning 76 Cleaning the Oximeter ... 76 Cleaning Preamp Cables... 77 Cleaning Sensors ... 77 Maintenance 78 Maintenance Intervals ... 78 Waste Electrical and Electronics Equipment (WEEE) ... 78 Fuse Replacement ... 79 AC Power Fuse... 79 Battery Maintenance ... 80 Disconnecting the Battery ... 80 Battery Power Fuse ... 80 Appendix 82 Symbols... 82 Symbols on the Oximeter Front Panel ... 82 Indicators on the Oximeter Display Screen... 82 Symbols near Oximeter Accessory Connections ... 84 Symbols on Oximeter... 84 Symbols on Oximeter Packaging... 84 Symbols on Sensor Packaging ... 85 Symbols on Preamp Cables ... 85 Location of Laser Labels... 86 Warranty Policy ... 87 FORE-SIGHT Oximeters ... 87 Oximeter Configuration Record ... 89 Specifications ... 89 Accessories ... 92 Oximeter ... 92 Re-order No: ... 92 FORE-SIGHT Sensor Selection Guide ... 93 Other Accessories and Options ... 93 Philips IntelliVue/VueLink Accessories and Options ... 94
21-22-0217 Rev 14
Page 11
FORE-SIGHT Oximeter, MC-2000 Series
Figures Figure 1: Patient Environment... 22 Figure 2: Front View... 24 Figure 3: Rear View ... 25 Figure 4: Clockwise and counterclockwise directions ... 26 Figure 5: Major Areas of Display... 28 Figure 6: Patient Menu... 29 Figure 7: New Patient/Case Menu ... 30 Figure 8: Cleared Patient - Patient Parameter Menu ... 30 Figure 9: Quick Start Case – Enter Patient/Case info Menu... 31 Figure 10: Profiles Menu ... 32 Figure 11: View Menu ... 32 Figure 12: Auto Scale View... 33 Figure 13: Event Menu... 33 Figure 14: Setup Menu... 34 Figure 15: Help Menu... 34 Figure 16: Help Menu - Display at System Startup? ... 34 Figure 17: About Menu... 35 Figure 18: Default Keyboard Layout ... 35 Figure 19: Extended Keyboard Layout... 36 Figure 20: Connecting the Preamp Cable... 40 Figure 21: Disconnecting the Preamp Cable ... 41 Figure 22: Connecting the Sensor to Preamp Cable ... 43 Figure 23: Enter Patient/Case info Menu ... 44 Figure 24: On / Standby Key... 48 Figure 25: Sensor Start / Restart Key ... 49 Figure 26: Alarm Limits Menu ... 50 Figure 27: Preference Menu ... 52 Figure 28: Brightness Menu ... 52 Figure 29: Auto Dim Menu ... 53 Figure 30: Languages Menu ... 54 Figure 31: Ports Menu... 55 Figure 32: Port Setup Menu ... 55 Figure 33: Date & Time Menu ... 58 Figure 34: Positioning Event cursor ... 60 Figure 35: Event Menu... 61 Figure 36: Vertical Position of Event ... 62 Figure 37: Horizontal Position of Event... 63 Figure 38: Final Position of Event ... 63 Figure 39: Message: Saving Pease wait… ... 64 Figure 40: Event Action... 64 Figure 41: Start FS Data Collection Reminder... 70 Figure 42: Stop FS Data Collection Reminder ... 70 21-22-0217 Rev 14
Page 12
FORE-SIGHT Oximeter, MC-2000 Series
Figure 43: FS Data Review Menu ... 71 Figure 44: Single Channel FS Data Review Menu... 71 Figure 45: Save to USB Menu - Initial... 72 Figure 46: Saving to USB Menu - Progress ... 73 Figure 47: Saving to USB Menu - Completed ... 74 Figure 48: USB Memory File - Example... 74 Figure 49: Save to USB Menu - Status Message ... 75 Figure 50: Snapshot to USB Menu ... 75 Figure 51: AC Fuse Placement ... 79 Figure 52: Battery Fuse Placement... 81 Figure 53: Location of Internal Laser Labels... 86
Tables Table 1: User Messages ... 66 Table 2: User Messages on Philips IntelliVue... 69 Table 3: USB Status Messages ... 74
21-22-0217 Rev 14
Page 13
FORE-SIGHT Oximeter, MC-2000 Series
Safety
Safety The operator must comply with the following Warnings, Cautions, and Notes to guarantee safe operation of the Oximeter. Additional Warnings, Cautions, and Notes, which apply to specific parameters, are listed in the sections that pertain to each parameter.
Indications for Use The FORE-SIGHT® Oximeter, Model MC-2000 Series is indicated for the continuous noninvasive monitoring of regional hemoglobin oxygen saturation of blood in the brain and skeletal muscle. It is intended for use in any individual at risk for reduced-flow or noflow ischemic states. When used with Large sensors, the FORE-SIGHT Oximeter is indicated for use on the brain of adults and children over 40 kg. When used with Medium sensors, the FORE-SIGHT Oximeter is indicated for use on the brain of small adults and children between 4 kg and 80 kg. When used with Small sensors, the FORE-SIGHT Oximeter is indicated for use on the brain of infants and neonates ≤ 8 kg. When used with Medium sensors, the FORE-SIGHT Oximeter is indicated for use on skeletal muscle of infants, children and adolescents between 5 and 50 kg. The prospective clinical value of data from the FORE-SIGHT Oximeter has not been demonstrated in disease states and these data should not be used as a sole basis for diagnosis or therapy.
Contraindications • The FORE-SIGHT sensor is contraindicated for use on patients with physical site area too limited for proper sensor placement. • The FORE-SIGHT sensor is contraindicated for use on patients with allergic reactions to electrode adhesive. • The sensor site must be checked at least every eight hours; and if the circulatory condition or skin integrity has deteriorated, the sensor should be applied to a different site. • Do not adhere sensors to underdeveloped, immature, compromised, or healing skin. • No other contraindications are known at this time.
21-22-0217 Rev 14
Page 14
FORE-SIGHT Oximeter, MC-2000 Series
Safety
Installation and Setup Warning: The Oximeter is defibrillator-proof. It may remain attached to the patient during defibrillation, but the readings may be inaccurate during use and less than twenty (20) seconds thereafter. Warning: The Oximeter is intended only as an adjunct in patient assessment. It must be used in conjunction with clinical signs and symptoms. Warning: Do not rely exclusively on the audible alarm system for patient monitoring. Adjustment of alarm volume to a low level during patient monitoring may result in a hazard to the patient. Remember that the most reliable method of patient monitoring combines close personal surveillance with correct operation of monitoring equipment. Warning: Do not place the Oximeter face against a surface. This will cause the alarm to be muffled. Warning: Do not place the Oximeter back against a surface. This will block the fan and cause the unit to overheat, shutting it off. Warning: Do not place the Oximeter or accessories in any position that might cause it to fall on the patient. Warning: Do not lift or pull the Oximeter by any cable as it could cause the Oximeter to fall on the patient. Warning: Do not place the Oximeter where the controls can be changed by the patient. Warning: Do not use the Oximeter for any purpose other than specified in this manual. Doing so will invalidate the Oximeter’s warranty. Warning: Do not connect more than one patient to an Oximeter. Warning: Leakage Current Test – The interconnection of auxiliary equipment, including a patient monitor or other patient-connected equipment, with this device may increase the total leakage current. When interfacing with other equipment, qualified biomedical engineering personnel must perform a test for leakage current before using it with patients. Serious injury or death could result if the leakage current exceeds applicable standards. Warning: The Oximeter is to be operated by qualified personnel only. This manual, accessory directions for use, all precautionary information, and specifications should be read before use. Warning: Do not expose the Oximeter to excessive moisture such as direct exposure to rain. Excessive moisture can cause the Oximeter to perform inaccurately or fail. Warning: Do not place containers containing liquids on or near the Oximeter. Liquids spilled on the Oximeter may cause it to perform inaccurately or fail. Warning: Patient Safety – If a Sensor is damaged in any way, discontinue use immediately. 21-22-0217 Rev 14
Page 15
FORE-SIGHT Oximeter, MC-2000 Series
Safety
Warning: The Oximeter is not “Category AP or APG Equipment.” Warning: Explosion Hazard – Do not use the Oximeter in the presence of a flammable anesthetic mixture with air or with oxygen or nitrous oxide. Warning: Electromagnetic Compatibility (EMC) – The equipment needs special precautions if it is placed close to a strong transmitter such as X-ray equipment, MRI devices, TV, AM/FM radios, police/fire stations, an amateur (“ham”) radio operator, an airport, or a cellular phone. Their signals could interfere with the Oximeter, which may result in disruption of performance of this device or prevent the clear reception of signals by the Oximeter. This equipment has been tested and found to comply with the limits for medical devices to the EN 60601-1-2: 2002, and Medical Device Directive 93/42/EEC. These limits are designed to provide reasonable protection against harmful interference in a typical medical installation. This equipment generates, uses, and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to other devices in the vicinity. However, there is no guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interference to other devices, which can be determined by turning the equipment off and on, the user is encouraged to try to correct the interference by one or more of the following measures: • Reorient or relocate the receiving device. • Increase the separation between the devices. Consult the manufacturer for help. Warning: If the integrity of the protective earth conductor is in doubt, the unit may be operated from the internal batteries by disconnecting the AC line cord completely from the unit. Warning: Sensors must be optically connected before lasers can be turned on. Failure to do this may result in inaccurate readings. Warning: Do not place Sensor over poorly perfused tissues. Warning: Avoid uneven skin surfaces for best adhesion. Warning: In the event that the location of the selected tissues cannot be palpated or visualized, confirmation by ultrasound or xray is recommended. Warning: Do not place sensor over sites with ascites, cellulitis or edema. Warning: Prolonged periods of pressure (such as taping over the Sensor or the patient lying on a Sensor) transfers weight from the Sensor to the skin, which can injure skin and reduce Sensor performance.
21-22-0217 Rev 14
Page 16
FORE-SIGHT Oximeter, MC-2000 Series
Safety
Caution: The Battery fuse must be installed for the unit to operate with the internal batteries. If the unit is not to be used for periods greater than 1 week, the battery should be disconnected (see Disconnecting the Battery on page 80). Caution: The USB connector accommodates a CASMED USB memory stick; do not connect any other USB type device or cable. Caution: Qualified biomedical engineering personnel only must interface monitoring equipment with other types of medical equipment. Be certain to consult manufacturers’ specifications to maintain safe operation. Caution: Measurements may be affected in the presence of strong electromagnetic sources such as electro-surgery equipment. Note: The Oximeter is designed for continuous operation. Note: The Oximeter is suitable for use in the presence of electrosurgery; however, measurements may be inaccurate during use of such equipment. Note: The Oximeter can remain connected to the patient during cardio defibrillation. All applied parts are “Type BF Defibrillation Proof.” The Oximeter has been designed to promote patient safety. All equipment parts are protected against the effects of the discharge of a defibrillator. No separate actions are required when using this equipment with a defibrillator.
Device Handling Warning: To ensure patient safety, do not place the Oximeter in any position that might cause it to fall on the patient. Warning: Do not lift or pull the Oximeter by any cable as it could cause the Oximeter to fall on the patient. Warning: To avoid electric shock or device malfunction, liquids must not be allowed to enter the device. If liquids have entered a device, take it out of service and have it checked by a service technician before it is used again. Warning: The Oximeter provides “DRIP-PROOF” level of protection from ingress to moisture. Warning: Do not place liquids on top of the Oximeter. Do not immerse the Oximeter or power cord in water or any liquid. Warning: Do not gas sterilize or autoclave the Oximeter. Warning: After removal of covers, connectors, etc., do not touch any part of non-medical electrical equipment and the patient at the same time. Warning: Where the integrity of the external protective conductor in the installation or its arrangement is in doubt, EQUIPMENT shall be operated from its INTERNAL ELECTRICAL POWER SOURCE. 21-22-0217 Rev 14
Page 17
FORE-SIGHT Oximeter, MC-2000 Series
Safety
Warning: Isolation of product from mains can only be achieved by removal of external power cord. Warning: Route and secure all cables away from patient’s throat to reduce the possibly of strangulation. Caution: Pressing the front panel keys with a sharp or pointed instrument may permanently damage the membrane. Press the keys using only your finger. Caution: If the Oximeter is accidentally wetted, take it out of operation. It should be thoroughly dried. Note: There are no known risks with common disposal of equipment or accessories; however, the disposing of accessories should follow in accordance with local hospital policies. The user should ensure these policies do not conflict with any local, state, or federal guidelines.
Safety Checks Warning: Do not, under any circumstances, perform any testing or maintenance on the Oximeter or power cord while the unit is being used to Monitor a patient. Unplug the power cord before cleaning or servicing the Oximeter. The operator should not perform any servicing except as specifically stated in this manual. Warning: The functions of the alarm system for monitoring of the patient must be verified at regular intervals. Warning: Periodically, and whenever the integrity of the product is in doubt, test all functions. Warning: Do not use a frayed or damaged power supply cord or any accessory if you notice any sign of damage. Contact CASMED for assistance. Refer to Contact Addresses on page 2 for e-mail and phone number information. Warning: The use of accessory equipment not complying with the equivalent safety requirements of this equipment may lead to a reduced level of safety of the resulting system. Consideration relating to the choice shall include: - Use of the accessory in the patient environment. - Evidence that the safety certification of the accessory has been performed in accordance to the appropriate IEC 60601-1 and/or IEC 60601-1-1 harmonized national standard. Caution: Inspect the Oximeter, cables, and Sensors for damage prior to operation. If any damage is noted, the Oximeter should not be used until it has been serviced. Only personnel authorized to do so by CASMED should repair the Oximeter. Caution: If the Oximeter fails to respond, do not use it until the situation has been corrected by qualified CASMED personnel.
21-22-0217 Rev 14
Page 18
FORE-SIGHT Oximeter, MC-2000 Series
Safety
Monitoring Warning: Conductive Connections – Extreme care must be exercised when applying medical electrical equipment. Many parts of the human–machine circuit are conductive, such as the Sensors, connectors, electrodes, and transducers. It is very important that these conductive parts do not come into contact with other grounded, conductive parts when connected to the isolated patient input of the device. Such contact would bridge the patient's isolation and cancel the protection provided by the isolated input. In particular, there must be no contact of the neutral electrode and ground. Warning: Do not come into contact with patients during defibrillation. Otherwise serious injury or death could result. Warning: If the accuracy of any value displayed on the Oximeter is questionable, determine the patient's vital signs by alternative means. Verify that all equipment is working correctly. Warning: Only use CASMED-supplied Sensors with this Oximeter. The use of unprotected Sensors creates the potential for making an electrical connection to ground or to a high voltage power source which can cause serious injury or death to the patient. Warning: As with all medical equipment, carefully route and secure all cables to reduce the possibility of patient entanglement or strangulation. Warning: Always remove Sensors from the patient and completely disconnect the patient from the Oximeter before bathing the patient. Warning: Before using Sensors for the first time, please read the instructions for use provided with the Sensors. Warning: The Sensor site must be inspected at least every eight hours; to ensure adequate adhesion, circulation, and skin integrity If the circulatory condition or skin integrity has deteriorated, the Sensor should be applied to a different site. Warning: Interfering substances: Carboxyhemoglobin may erroneously increase the readings. The level of increase is approximately equal to the amount of carboxyhemoglobin present. Dyes or any substance containing dyes that change usual blood pigmentation may cause erroneous readings. Other factors that may affect the accuracy of measurement include: hemoglobinopathies, myoglobin, anemia, pooled blood under the skin, interference from a metal plate or other foreign object in sensor path, intravascular dyes, Bilirubinemia, tattoos or birthmarks. Warning: Do not place Sensor over poorly perfused tissues. Warning: For patients experiencing complete bilateral ECA occlusion, measurements may be lower than expected.
21-22-0217 Rev 14
Page 19
FORE-SIGHT Oximeter, MC-2000 Series
Safety
Warning: To prevent damage, do not soak or immerse the Sensors in any liquid solution. Do not attempt to sterilize. Warning: Intravascular dyes or externally applied coloring may lead to inaccurate measurements. Warning: Elevated levels of carboxyhemoglobin (COHb) may lead to inaccurate measurements. Warning: Elevated levels of methemoglobin (MetHb) will lead to inaccurate measurements. Warning: Sensors are not sterile and therefore should not be applied to sites with compromised skin integrity. Exercise caution when applying Sensors to a site with compromised skin integrity. Applying tape or pressure to such a site may reduce circulation and/or cause further skin deterioration. Warning: Misapplied Sensors or Sensors that become partially dislodged may cause either over- or under-reading of oxygen saturation. Warning: Failure to apply Sensors properly may cause incorrect measurements. Warning: Do not modify or alter the Sensor in any way. Alterations or modification may affect performance and/or accuracy. Warning: If the patient’s skin temperature is greater than 39.2 °C, to prevent possible damage to the patient’s skin, assess the sensor site more frequently Warning: Do not position Sensor under the weight of the patient. Warning: Prolonged periods of pressure on the Sensor can injure skin and reduce Sensor performance. Warning: Do not apply prolonged pressure to the Sensor. Use only approved FORE-SIGHT Headband or HOLD-TIGHT devices with non-adhesive Sensor. Caution: Use only accessories and Sensors approved by CASMED to ensure patient safety and to preserve the integrity, accuracy, and electromagnetic compatibility of the Oximeter. Caution: Electrocautery – To prevent unwanted skin burns; apply electrocautery electrodes as far as possible from all other Sensors, a distance of at least 15 cm (6 in.) is recommended.
21-22-0217 Rev 14
Page 20