Cincinnati Sub-Zero
Blanketrol CoolRepeat Instructions for Use Rev 2
Instructions for Use
9 Pages
Preview
Page 1
English
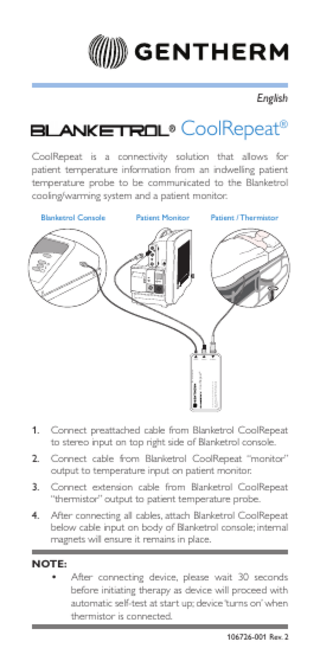
CoolRepeat® CoolRepeat is a connectivity solution that allows for patient temperature information from an indwelling patient temperature probe to be communicated to the Blanketrol cooling/warming system and a patient monitor. Patient / Thermistor
CAUTION: When using Accutrol Catheter please refer to IFU.
CONSOLE BATTERY INDICATOR
MONITOR
CoolRepeat®
NOTE: UNPLUG ALL CONNECTIONS WHEN NOT IN USE. REPLACE BATTERY WHEN LED IS FLASHING RED.
106724-001 Rev 1
Patient Monitor
THERMISTOR
Blanketrol Console
1.
Connect preattached cable from Blanketrol CoolRepeat to stereo input on top right side of Blanketrol console.
2.
Connect cable from Blanketrol CoolRepeat “monitor” output to temperature input on patient monitor.
3.
Connect extension cable from Blanketrol CoolRepeat “thermistor” output to patient temperature probe.
4.
After connecting all cables, attach Blanketrol CoolRepeat below cable input on body of Blanketrol console; internal magnets will ensure it remains in place.
NOTE: • After connecting device, please wait 30 seconds before initiating therapy as device will proceed with automatic self-test at start up; device ‘turns on’ when thermistor is connected. 106726-001 Rev. 2
Blanketrol® CoolRepeat®
WARNINGS: • Blanketrol CoolRepeat must be used with a Blanketrol system. •
Once thermistor and cables are connected, verify that temperature consistently displays on console and monitor.
Instructions for Use
Graphic Symbols for Medical Device Labeling Attention: See Instructions for Use
•
Discontinue use if LED on top of unit is not illuminated when connected to thermistor.
N M
•
Inspect all components prior to use. Do not use any component that appears damaged.
LATEX
•
For unit to function, please make sure battery is installed and all cables are properly connected.
•
Make sure to disconnect thermistor cable after use to avoid depleting battery as device ‘turns on’ when thermistor/patient temperature cable is connected.
•
Only change battery when device is not connected to patient.
•
Remove battery from device if not likely to be used for more than 6 months.
•
Avoid movement of console when device is connected to minimize loads on connecting cables.
•
Avoid contact with ionic or corrosive fluids.
Date of Manufacture Manufacturer Does not contain natural rubber latex Separate Collection for Electrical and Electronic Equipment Do not use if box is damaged Keep Away From Heat Store in a Cool Dry Place Type CF, Defibrillation Proof, Applied Part Direct Current Recyclable Paper Product
U.S. Federal law restricts this device to sale on or by the order of a physician.
60°C
-20°C
l
Temperature Limitation Type B Equipment U.S. Federal law restricts this device to sale by or on the order of a physician
Copyright © 2021 ZOLL Medical Corporation. All rights reserved. ZOLL®, CoolRepeat® are trademarks or registered trademarks of ZOLL Medical Corporation and/or its subsidiaries in the United States and/or other countries. All other trademarks are the property of their respective owners. 2
1
QTY Quantity
3
Blanketrol® CoolRepeat®
Instructions for Use
Battery
Specifications
Indicator
Power Requirements – DC Battery
Indicator light on the top edge of the device emits light patterns to indicate several conditions:
9V
Flashing Green – Normal Operation when unit is ‘on’ (thermistor cable is plugged in). Flashing Red – Low Battery; replace battery. Solid Red – Fault State; attempt to re-set device by disconnecting and re-attaching cables. If Solid Red light pattern persists, discontinue use, there is a problem with the device.
.
NOTE: Lithium-ion 9V battery recommended for maximum hours of operation, but can also use standard 9V Alkaline battery.
Operating Limits Temperature Range: 10°C to 40°C.
Replacement
Humidity Range: 20% to 60% (non-condensing).
To replace battery, unplug all cables from the device and remove from console. Slide open battery compartment door, carefully disconnect battery and attach new battery.
Atmospheric Pressure: 700 hPa to 1060 hPa.
Suggested Battery: Ultralife 9 Volt Lithium; Part# U9VL-FP.
Disposal Batteries need to be disposed of in accordance with local ordinances.
Maintenance Complete annual accuracy evaluation by connecting device to thermistor simulator and verify repeated resistance on outputs. No user serviceable parts. Surface of the device should be cleaned regularly with soft cloth or paper towel dampened with warm water and a mild, non-abrasive, non-staining hospital disinfectant or detergent.
4
Input Patient Temperature Range: 0°C-45°C. Output Accuracy: 0.1 °C in the range of 32°C to 42°C; 0.2 °C outside this range.
Shipping/Storage Limits Temperature Range: -20 °C to +60 °C. Humidity Range: 20% to 95% (non-condensing). Atmospheric Pressure: 500 hPa to 1060 hPa.
Physical Dimensions Size: 3 H x 1 D x 5.5 W inch; 7.6 x 2.5 14cm. Weight: 7 ounces (200 grams).
Classification of Equipment Type B, Applied Part, Internally powered. Mode of Operation: continuous.
5
Blanketrol® CoolRepeat®
Instructions for Use
Device Inputs One (1) input for YSI-400 compatible temperature cable. One (1) output from YSI-400 compatible temperature cable. Blanketrol Part #8700-000833-01 can be used for both connectors. Has been designed to be compatible with YSI 400 series thermistor input monitors with less than 330 microamperes DC only (non pulsing) thermistor drive current at 7500 ohms. Only connect to patient monitors that comply with IEC60601-1.
Catalog Number Blanketrol CoolRepeat catalog number is 8700-001026-01. For pricing information or to place an order, please contact the Gentherm Customer Service department at +1-513-772-8810.
Guidance and Manufacturer’s Declaration Electromagnetic Emissions The Blanketrol CoolRepeat Console is intended for use in the electromagnetic environment specified below. The customer or the user of the Blanketrol CoolRepeat device should assure that it is used in such an environment.
Emissions Tests RF emissions
Compliance Group 1
The Blanketrol CoolRepeat device, Model 25-01 uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment.
Class A
The Blanketrol CoolRepeat device, Model 25-01 is suitable for use in all establishments other than domestic and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes.
CISPR 11 RF emissions CISPR 11 Harmonic emissions
Electromagnetic Environment Guidance
Class A
IEC 61000-3-2 Voltage fluctuations/flicker emissions IEC 61000-3-3 6
Complies
7
Blanketrol® CoolRepeat®
Instructions for Use
Electromagnetic Immunity The Blanketrol CoolRepeat device, Model 25-01 is intended for use in the electromagnetic environment specified below. The customer or the user of the Blanketrol CoolRepeat device, Model 25-01 should assure that it is used in such an environment. Immunity Test
IEC 60601 Test Level
Compliance Level
Electromagnetic Environment Guidance
Electrostatic discharge (ESD) IEC 61000-4-2
±6 kV contact ±8 kV air
±6 kV contact ±8 kV air
Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%.
Electrical fast transient/burst IEC 61000-4-4
±2 kV for power supply lines ±1 kV for input/ output lines
±2 kV for power supply lines ±1 kV for input/ output lines
Mains power quality to Blanketrol console should be that of a typical hospital environment. The Blanketrol CoolRepeat does not possess a CD power port and the interface cables do not exceed 3 meters in length.
Surge IEC 61000-4-5
±1 kV differential mode ±2 kV common mode
±1 kV differential mode ±2 kV common mode
Mains power quality should be that of a typical commercial or hospital environment.
Voltage dips, short interruptions and voltage variations on power supply input lines IEC 61000-4-11
<5% UT (>95% dip in UT) for 0,5 cycle 40% UT (60% dip in UT) for 5 cycles 70% UT (30% dip in UT) for 25 cycles <5% UT (>95% dip in UT) for 5 sec
<5% UT (>95% dip in UT) for 0,5 cycles 40% UT (60% dip in UT) for 5 cycles 70% UT (30% dip in UT) for 25 cycles <5% UT (>95% dip in UT) for 5 sec
Mains power quality should be that of a typical commercial or hospital environment. If the user of the Blanketrol CoolRepeat device requires continued operation during power mains interruptions, it is recommended that the Blanketrol console be powered from an uninterruptible power supply.
Power frequency (50/60 Hz) magnetic field
3 A/m
3 A/m
Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment.
IEC 61000-4-8 NOTE: UT is the AC mains voltage prior to application of the test level. 8
9
Blanketrol® CoolRepeat®
Instructions for Use
Electromagnetic Immunity (continued) IEC 60601 Test Level
Compliance
Conducted RF IEC 61000-4-6
Immunity Test
3 Vrms 150 kHz to 80 MHz
3 Vrms
Radiated RF IEC 61000-4-3
3 V/m 80 MHz to 2,5 GHz
3 V/m
Level
Electromagnetic Environment Guidance Por table and mobile RF communications equipment should be used no closer to any part of the Blanketrol CoolRepeat device, Model 25-01, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter. Recommended separation distance:
where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in meters (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey,a should be less than the compliance level in each frequency range.b Interference may occur in the vicinity of equipment marked with the following symbol: a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicated theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measure field strength in the location in which the Blanketrol CoolRepeat device is used exceeds the applicable RF compliance level above, the Blanketrol CoolRepeat device should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the Blanketrol CoolRepeat device. b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
NOTE: 1. At 80 MHz and 800 MHz, the higher frequency range applies. 2. These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. 10
11
Blanketrol® CoolRepeat®
Instructions for Use
Recommended Separation Distances Recommended Separation Distances Between Portable and Mobile RF Communications Equipment and the Blanketrol CoolRepeat Device. The Blanketrol CoolRepeat device, Model 25-01, is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the Blanketrol CoolRepeat device, Model 25-01 can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the Blanketrol CoolRepeat device, Model 2501, as recommended below, according to the maximum output power of the communications equipment.
Separation Distance According to Frequency of Transmitter (m) Rated Maximum Output Power of Transmitter (W)
150 KHz to 80 MHz
80 MHz to 800 MHz
800 MHz to 2,5 GHz
0.01
0.12
0.12
0.23
0.1
0.38
0.38
0.73
1
1.2
1.2
2.3
10
3.8
3.8
7.3
100
12
12
23
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated using the equation applicable to the
frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE: 1. At 80 MHz and 800 MHz, the higher frequency range applies. 2. These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
12
13
Blanketrol® CoolRepeat®
This page intentionally left blank.
14
Instructions for Use
This page intentionally left blank.
15
Distributed by: Gentherm Medical 12011 Mosteller Road Cincinnati, OH 45241 1-513-772-8810 www.gentherm.com ZOLL International Holdings B.V. Newtonweg 18 6662 PV ELST The Netherlands Tel: 31 481 366410
M
ZOLL Circulation Inc. 2000 Ringwood Avenue San Jose, CA 95131 U.S.A. t: +1-408-541-2140