Compumedics
Compumedics DWL Doppler Systems
Compumedics DWL Multi-Dop T digital Operating Instructions Rev 2 March 2017
Operating Instructions
113 Pages
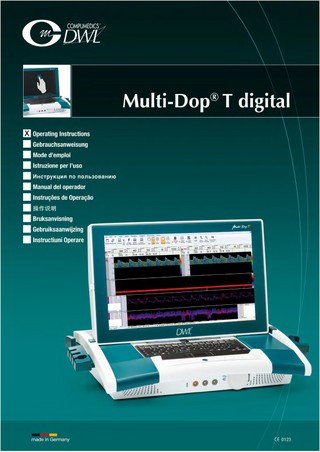
Preview
Page 1
Operating Instructions ®
Multi-Dop T digital valid for Multi-Dop® T devices from serial number MDT-2002 on Revision 2
0123
Art.-Nr. 9038E-2
14 March 2017
Compumedics Germany GmbH
Revision 0: Revision 1: Revision 1:
24 June 2015 7 September 2015 14 March 2017
These operating instructions comprise the description of the hardware and contains other useful pieces of information. The software program QL is described in separate operating instructions.
These operating instructions are valid from 14 March 2017 on and expire when new ones are released.
Copyright: The contents of these operating instructions are the property of Compumedics Germany GmbH and are protected by copyright. Any reproduction of the instructions, in whole or in part, is prohibited. Subject to change without notice. Multi-Dop and DWL are registered trademarks of Compumedics Germany GmbH. Pentium and Core 2 Duo are registered trademarks of Intel Corp. Windows is a registered trademark of Microsoft. CaviCide is a registered trademark of Metrex. US 6 344 024
IV
EP 0 981 764 MDTdig e, Rev. 2
Compumedics Germany GmbH
Multi-Dop® T digital digital – portable – complete Thoroughly ergonomical design and time proven DWL® Doppler quality are outstanding features of our portable Multi-Dop® T digital complete Doppler system. With 1, 2, 2+2.5*, 4, 8 and 16 MHz Doppler frequencies the MultiDop® T digital is perfectly equipped for transcranial, extracranial, peripheral and microvascular examinations. The modular design facilitates an upgrade of the software at any time from routine applications to unilateral or bilateral monitoring, special function tests emboli detection and differentiation with the maximum of efficiency. An integrated 17.3“ (43.9 cm) TFT colour multi-touch display, integrated detachable Bluetooth keyboard, high quality loudspeakers, multi-function remote control with integrated optical mouse and user friendly software guarantee optimal working conditions. Full Digital Doppler Technology The traditionally high quality DWL® Doppler with digital Doppler technology and Doppler M-Mode meets highest demands and exceeds all previous requirements for clinical routine and monitoring examinations. The integrated digital technology provides a maximal signal quality and total access to raw data for complete external signal analysis by means of ASCII export. Doppler M-Mode The Doppler M-Mode makes it possible to simultaneously receive and analyse Doppler signals from a predefined range of depths. The Doppler M-Mode display shows the intensity, direction and depth information of this range of depths continuously and in real-time. It provides an excellent graphical display of the blood flow in all vessels located in the sound beam. The Doppler M-Mode display allows moving through the entire depth range and thus easily selecting the best Doppler signals and most relevant clinical data. Use of the Doppler M-Mode for emboli detection allows an observation of an embolus as it passes through various depths. The Doppler M-Mode window contains the signals of up to 8,000 (micro) gates. The number is calculated from the formula: 1
𝑁𝑔𝑎𝑡𝑒𝑠 = 𝑁𝑐ℎ ∗ 𝑃𝑅𝐹 /µ𝐺 Nch = number of channels (1 or 2) µG = the smallest calculated gate (microgate), here: 0.25µs PRF = scale in Hz (smallest scale setting = 1000 Hz)
* The 2+2.5 MHz probe can only by used together with the upgrade emboli differentiation which is not available in the USA. ** The hardware dependent microgate is used internally to calculate the M Mode window and the normal depth gates that are displayed. MDTdig e, Rev. 2
V
Compumedics Germany GmbH QL Software The user friendly QL software with intuitive user interface is based on a Windows® operating system. The modular design facilitates an upgrade of the software at any time from routine applications to unilateral or bilateral monitoring, special function tests, emboli detection and differentiation with the maximum of efficiency. The Doppler M-Mode makes it possible to simultaneously receive and analyse Doppler signals from a predefined range of depths. The Doppler M-Mode display shows the intensity, direction and depth information of this range of depths continuously and in real-time. It provides an excellent graphical display of the blood flow in all vessels located in the sound beam. The Doppler M-Mode display allows moving through the entire depth range and thus easily selecting the best Doppler signals and most relevant clinical data. Use of the Doppler M-Mode for emboli detection allows an observation of an embolus as it passes through various depths. Advantages of the Multi-Dop® T digital Portable complete Doppler system High quality DWL® Doppler Full digital Doppler system Bluetooth keyboard with integrated mouse pad Bilateral Doppler M-Mode (32 channels each) Up to 400 gates Multi-touch display with tablet function Upgradeable with optional software modules up to unilateral and bilateral monitoring diagnostic with special function tests and emboli detection and differentiation* Intuitive user interface – simple to navigate and freely configurable Windows® Embedded 8.1 Industry Pro Network connectivity Analog in- and outputs With fast 128 GB SSD memory module Optional: integrated CO2 module
VI
MDTdig e, Rev. 2
Compumedics Germany GmbH
Cautions Federal Law (of the USA) restricts this device to be used by or on the order of a physician!
Do not exceed an ISPTA of 17 mW/cm2 when scanning through the eye!
Do not exceed an ISPTA of 94 mW/cm2 when scanning through the foramen magnum, burr holes and infant skulls!
Minimise ultrasound exposure at all times by using the lowest power settings!
For monitoring, switching probes on only during critical phases of surgery is preferred over continuous monitoring!
When using 16 MHz probes invasively on high-infectious risk patients (Creutzfeld-Jakob), destroy the probes afterwards. Do not use them again.
This device is not intended for foetal use!
For USA only: This device is strictly for adolescent and adult studies!
MDTdig e, Rev. 2
! ! ! ! ! ! ! ! VII
Compumedics Germany GmbH
Table of Contents 1. General ... 1-1 Classification ... 1-1 How to Use These Operating Instructions ... 1-2 Explanation of Symbols and Markings Used ... 1-3 Safety Instructions ... 1-4 Safety Instructions for Monitoring ... 1-8 Information on Thermal Indices ... 1-9 Information on Network Safety ... 1-9 Error Messages... 1-9 Information about USB Flash Drives ... 1-9 Environmental Conditions ... 1-10 Conditions for Transport and Storage ... 1-10 Instructions on Disposal ... 1-11
2. The Design ... 2-1 Relocating Holders at the Side Panels ... 2-2 Connections at Rear Panel ... 2-3 Getting Started ... 2-4 System Power ... 2-5 Checking the Basic Functions ... 2-6 Maintenance ... 2-6 Ultrasound Probes ... 2-7 Standard probes (1, 2, 2+2.5*, 4, 8 MHz), handheld ... 2-7 Monitoring probes (2, 2+2.5, 4 MHz) ... 2-8 Cleaning and Maintenance of Probes ... 2-8 16 MHz Disposable Probe ... 2-10 Further Components and Accessories ... 2-11
3. Example of a Routine Measurement ... 3-1 Preparing a Measurement ... 3-1 Carrying Out a Measurement ... 3-2 Default Parameter Settings (Standard Probes) ... 3-3
4. Technical Information... 4-1 Technical Data ... 4-1 Configuration of Analog Input/Output Connectors (15 pins) ... 4-3 Potential Equalisation ... 4-3
VIII
MDTdig e, Rev. 2
Compumedics Germany GmbH Electrical Safety Test ...4-3 Declarations of Conformity and Guarantee ...4-6
5. Clinical Use and Toggle Mode ... 5-1 Clinical Use ...5-1 Introducing Toggle Mode ...5-2 Toggling Between CW and PW Operation ...5-2 CW & PW Ultrasound Probes ...5-4
6. System Care: Cleaning and Probe Care ... 6-1 Environmental Considerations ...6-1 Operating Rooms and Sterile Fields ...6-1 System Cleaning & Disinfecting (for USA) ...6-1 Cleaning, Disinfection, Sterilisation Instructions for Europe ...6-5 Device and Accessories (except probes) ...6-5 1, 2, 2+2.5, 4, 8 MHz Probes ...6-6 16 MHz Reusable Probes...6-7
Appendix A: Ultrasound Output Values and Clinical Accuracy ... A-1 Ultrasound Power Output Measurements ...A-1 Results According to FDA Guidelines ...A-1 Results According to IEC 60601-2-37 ...A-10 Basic Interaction between Ultrasound and Matter and Possible Bioeffects ...A-17 Information on Interpretation of Thermal Indices ...A-18 ALARA Principles ...A-19 Clinical Accuracy ...A-20
Appendix B: Information on EMC according to IEC 60601-1-2 ... B-1 ESD precautionary procedures ... B-1 ESD training ... B-2
Appendix C: Sample Examination Techniques ... C-1 Sample TCD Examination Procedures... C-1 Sample 16 MHz Intraoperative Examination Procedures (For USA Only) ... C-4
Appendix D: Built-in CO2 Module (Option) ... D-1 Safety Instructions ... D-1 Using the CO2 Module ... D-2 MDTdig e, Rev. 2
IX
Compumedics Germany GmbH
Appendix E: Accessories ... E-1 Probes ... E-5 Monitoring, MRI... E-6 16 MHz Probes... E-6 Adapters and Extension Cables for Monitoring and 16 MHz Probes ... E-6 DiaMon® Sets ... E-7 Headband Sets Size L/XL ... E-8 LAM Sets Standard ... E-10 LAM Sets Neurosurgery ... E-12 LAM Sets Carotid ... E-13 LAM Sets Mandibula ... E-14 Adhesive Sets ... E-16 Additional Software ... E-16 Disposables ... E-16
X
MDTdig e, Rev. 2
Compumedics Germany GmbH
1. General The Multi-Dop® T digital system is a medical ultrasound device for measuring the blood flow Intended use velocities in arteries and veins mainly transcutaneously. The 16 MHz probes can be used invasively. The Multi-Dop® T digital is certified by CE and meets the requirements of the 93/42/EEC - Compliance with Annex II.3 Medical Device Directive. It is designed to the standards IEC 60601-1 (ed.3):am1 IEC 60601-1-2 (ed.3):am1 IEC 60601-2-37 (ed.2) and complies with the guidelines issued by the German Association of Medical Insurance Companies. The device must only be used together with accessories of other manufacturers when Compumedics Germany (DWL) has permitted it explicitly. The only risk known so far when using ultrasound is a possible plaque ablation due to the Side effects and compression of the artery by the probe. risks Do not exceed an ISPTA of 17 mW/cm2 when scanning through the eye! Do not exceed an ISPTA of 94 mW/cm2 when scanning through the foramen magnum, burr holes and infant skulls! It is common practice to verify the results of a certain diagnosis method by another one prior Important to applying any treatment to the patient.
Classification - type of protection against electric shock: safety class 1 equipment
acc. to IEC 60601-1
- grade of protection against electric shock: ultrasound probes 1, 2, 2+2.5, 4, 8 MHz BF applied parts 16 MHz probes CF applied part - grade of protection against ingression of solid particles or fluids Device: IP 20 1, 2, 2+2.5, 4, 8 MHz probes: IP 64 16 MHz probes: IP 67 - grade of protection when using the devices while anaesthetics are present: devices must not be used in explosive atmospheres or in explosive mixtures of anaesthetics with oxygen or laughing gas. - approved sterilisation methods: reusable 16 MHz probes can be gas-sterilised 1, 2, 2+2.5, 4, 8 MHz probes MUST NOT be gas-sterilised - approved methods of usage: devices are suitable for continuous operation.
* In the following chapters only the basic identification number of each standard is given. MDTdig e, Rev. 2
1-1
Compumedics Germany GmbH - Pollution degree: 2 acc. to MDD 93/42/EEC
device of class IIa (according to rule 10)
according to UMDNS
15-957 16-272
device and accessories (except probes) probes
according to GMDN
61236 61422 61227 36970 61226
vascular ultrasound system control unit non-invasive vascular ultrasound system, line-powered invasive vascular ultrasound system all probes except 16 MHz 16 MHz probes
according to JMDN
4075900
How to Use These Operating Instructions These operating instructions have been written specifically with the user in mind. They are intended to help the operator to use the unit to its full potential. To avoid any operating errors please read these instructions carefully before using the unit.
!
1-2
The operating instructions should always be kept near the unit in a place where the operator can locate them quickly whenever required. Warning: This device is intended to be used by skilled personnel only!
These operating instructions comprise a description of the device, technical information and further important notes. A detailed description of the software which is installed on the device is contained in the “Operating Instructions QL Software”.
MDTdig e, Rev. 2
Compumedics Germany GmbH
Explanation of Symbols and Markings Used The symbols used on the device and additional parts are explained below. Caution! Follow operating instructions!
LAN 1 LAN 2
Ethernet connection analog IN port
applied part: type BF
analog OUT port
applied part: type CF
ESD warning symbol connectors for DWL® ultrasound probes
date of production
probe sockets for 1st channel
USB port
probe socket for 2nd channel
serial port
Stand-by key
DVI-I port
Communité Européene certified, notified body (TÜV Product Service GmbH, München)
IP 20 IP 64 IP 67
DPP 1 DPP 2
Display port Line in
CO2 connectors
Line out (headphones)
2 = protected against objects > 12.5 mm 6 = dust tight
microphone connector
0 = not protected against ingress of water 4 = protected against splashing water 7 = protected against short-time immersion
equipotentiality product name
VDE approved
trademark logo MET approved
CAUTION DISCONNECT INPUT POWER BEFORE SERVICING. REFER SERVICING ONLY TO
serial number article number
SERVICE PERSONNEL AUTHORIZED BY COMPUMEDICS
Compumedics Germany GmbH Josef-Schüttler-Str. 2 address of manufacturer 78224 Singen Germany info@dwl.de www.dwl.de MDTdig e, Rev. 2
UDI sticker
service note 100-240V~ 50-60Hz max. 130VA
12V max. 11A
power note
DC input
1-3
Compumedics Germany GmbH single use only
sterile, sterilised by radiation
batch number expiring date:
if red, sterile (by Gamma radiation)
The following symbols are used in the manual:
!
Warning: is used if damages to the device could occur in extreme cases. Notes of this kind must always be observed.
Important is used for important and useful information.
Safety Instructions Instructions on starting-up
Do not use the system without training by a Compumedics Germany (DWL) authorized dealer. Inform your dealer when the system arrives before opening the package. In order to avoid the risk of an electric shock, connect this device only to mains with a protective conductor. Together with this unit, use only accessories and devices recommended by Compumedics Germany (DWL). Do not install software or driver programs by other manufacturers on the device (with the exception of anti-virus software and other anti-malware software). The proper operation of the device cannot be guaranteed if other software programs are installed on the hard disk. When connecting an external device (e.g. printer) make sure that both devices are switched off. Medical electrical devices need special precautions regarding EMC and have to be installed and put into service according to the information provided in appendix B. Portable and mobile RF communications equipment can affect medical electrical devices. First establish all connections (remote control, printer, etc.) before connecting the device to the mains. All connectors and inputs not in use must be sealed with plastic caps. Otherwise they may be damaged by electro-statical discharges. The users are responsible that the medical system they create at their place complies with standard IEC 60601-1 section 16.
1-4
MDTdig e, Rev. 2
Compumedics Germany GmbH If the device is connected to a non-medical network, the additional medical network isolator must be used. If a non-medically approved accessory is connected to the device using a USB port, a USB isolator must be used. This device must not be altered without explicit permission of Compumedics Germany GmbH. The patient should not touch any metallic parts of the device, the applied parts or the accessories. The device should be positioned in a way that allows quick separation from the mains. Only DC voltage sources that comply with IEC 60601 (class 2, 2MOPP) are permitted to be connected to this device. Never pull the 16 MHz probe out of the adaptor, always pull the adaptor out of the socket. Disconnecting a probe should only be done in off-line mode. For disconnecting probes, never pull at the cable, always use the plug!
Instructions on operating the Before use, always inspect the ultrasound probes for visible cracks or damage. Never use system cracked or damaged probes. Contact your authorised Compumedics Germany (DWL) dealer for repair or replacement. When using the device near or in operating theatres take care to ensure a safe galvanic separation so that safety and function of other (probably life-saving) devices are not compromised. For the 16 MHz probes a special adaptor for digital devices is needed. The 16 MHz adaptor for analog Doppler devices by Compumedics Germany cannot be used with the Multi-Dop® T digital. When making transorbital measurements reduce the transmission power to a minimum. Since the probes can get very hot it is essential that long-lasting measurements (with the probe fixed to the patient) are only carried out with a TIC limit set to a maximum of 2.0. Loud noise can damage your sense of hearing and it is a nuisance to your environment. Make sure the ventilation slots are not covered or blocked in any way. During application of the device, only external devices that were explicitly specified by Compumedics Germany (DWL) are permitted to be connected to the USB connector. USB devices that have an own power supply are only permitted to be used with this device when they are IEC 60601 certified or when a USB isolator is used.
MDTdig e, Rev. 2
1-5
Compumedics Germany GmbH When applying the device to a patient, only external devices that were explicitly specified by Compumedics Germany (DWL) are permitted to be connected to the device. Compumedics Germany (DWL) cannot be held responsible for damages that occur when this rule is not observed. Only devices that comply with IEC 60601 or IEC 60950 are permitted to be connected to the analog In and analog Out connectors. Use only Compumedics Germany (DWL) probes. The probes may be damaged when used near a working defibrillator. The 16 MHz probe must always be used together with the adaptor. Materials that have an expiring date must not be used after this date but should be disposed of according to national guidelines. If the packaging of a sterile accessory is damaged, it must not be used. Only use ultrasound gel that doesn’t cause any damage to the probe heads. We recommend the usage of ultrasound gel provided by Compumedics Germany (DWL). All accessories that are connected to the system Multi-Dop® T digital have to comply at least with IEC 60950 or, preferably with IEC 60601. The leakage currents defined in IEC 60601-1 have to be observed with every system configuration. Since we cannot guarantee that the leakage currents are below the limits when using an external printer, the printer must not be in reach of patients. That means... ...acc. to IEC 60601-1 minimum distance to the side = 1.5 m minimum distance above = 2.5 m ...acc. to UL minimum distance to the side = 1.83 m minimum distance above = 2.29 m External devices out of the patient’s reach have to comply with relevant standards (e.g. IEC 60950:2005). Devices must not be used in explosive atmospheres or in explosive mixtures of anaesthetics with oxygen or laughing gas. Do not stand or sit on the device. Always quit QL software before pressing the stand-by key. The device goes to stand-by mode. To turn it off completely you have to disconnect the power cord but wait until the LED lights up blue.
1-6
MDTdig e, Rev. 2
Compumedics Germany GmbH When closing the device, take care that your fingers don’t get caught between the upper and bottom parts of the device. Instructions on cleaning and Instructions on cleaning and disinfection can be found in chapter 6 under the heading “System maintenance Care: Cleaning and Probe Care”. Never carry out maintenance work while the device is used on a patient.
Cleaning of system accessories (e.g. printer, remote control, etc.) must take place out of the patients’ reach, using normal cleaning agents or cleaning agents recommended by the manufacturer. The 1, 2, 2+2.5, 4 and 8 MHz probes are fitted with a PVC cable and must not be gas-sterilised or autoclaved. The 16 MHz probes can be gas-sterilised. Instructions on sterilisation are contained in chapter 6 System Care: Cleaning and Probe Care”. When using the probes on patients with infectious risks (e.g. Creutzfeld-Jakob), dispose of the reusable 16 MHz probes and do not reuse them. The 16 MHz disposable probes are delivered in sterile condition. Check the packaging prior to opening it. If the packaging is damaged, the probe is no longer sterile and must not be used. Dispose of it according to national guidelines. They cannot be sterilised. The device does not contain any maintainable parts. It should only be repaired by technicians authorised by Compumedics Germany (DWL) who can order service instructions from the Compumedics Germany (DWL) dealers. Once a year, a complete electrical check-up – independent of national regulations - should be carried out by a qualified person in accordance to the guidelines given on page 4-3. Disconnect the probe plug to separate the device completely from the mains.
MDTdig e, Rev. 2
1-7
Compumedics Germany GmbH
Safety Instructions for Monitoring
! !
1-8
The ultrasound probes convert electrical energy to acoustic energy. Each time energy is converted from one form to another, part of it is inevitably converted to heat. This results in an increase of the temperature of the probes. When doing monitoring, the probes are fixed to the same position for quite some time. Warning: The following instructions must always be observed. •
Increase the gain setting to the maximum value and reduce the power to a setting as low as possible!
•
Carry out monitoring only during critical phases of the surgery.
•
The pressure the probes apply to the patient’s skin should not be too high since a high pressure reduces blood circulation.
•
Always apply plenty of ultrasound gel which ensures a good transduction of the ultrasound. Apply fresh gel several times during the monitoring.
•
Check the temperature of the probes and the skin under and around the probes regularly.
Warning: Patients who suffer from poor blood circulation e.g. diabetics or patients supplied with heart-lung machines, or patients with an increased body temperature, have to be observed meticulously.
MDTdig e, Rev. 2
Compumedics Germany GmbH
Information on Thermal Indices There are several different thermal indices (TI). For transcranial Doppler sonography (1, 2 and 2+2.5 MHz) the TIC is the most important one. For peripheral measurement, using 4 and 8 MHz, the TIB and TIS indices are important. The mechanical index is not used since mechanical effects (cavitation) can be neglected. The indices do not provide safety limits. What the indices do provide is an indication of the conditions which are more likely than others to produce thermal effects. The modelling for predicting TI assumes some cooling by blood perfusion. For applications where poorly perfused tissues are expected (e.g. diabetics), the TI should be maintained at a lower value (< 1.0). When insonating tissue known to be well perfused, a higher TI value can be tolerated (> 2.0). You can find more information on that in Appendix A on pages A-16 through A-18.
Information on Network Safety The device features two Ethernet ports and can be connected to a network. Provided the configuration allows for it, the device could be communicating in the public world wide web. In this case we strongly recommend that you use anti-virus software and contact your network administrator for further protective measures.
Error Messages All possible error messages of the QL software are explained in the Operating Instructions of the QL Routine software. For information about error messages of the Windows@ operating system, refer to Microsoft under Support or Technet.
Information about USB Flash Drives When you want to store data for a longer time on a USB flash drive, you should only use flash drives that are explicitely designed for long-term storage or those that use SLCs (Single Layer Chips). USB flash drives with Multi Layer or Triple Layer Chips (MLC, TLC) are not reliable when data are stored for a longer time. Data loss could occur.
MDTdig e, Rev. 2
1-9
Compumedics Germany GmbH
Environmental Conditions The device shall only be operated under the following conditions. temperature: +10°C to +40°C (+50°F to +104°F) ambient pressure: 700 hPa to 1060 hPa relative humidity: 30 % to 75% max. operating altitude: 3000 m The device should be positioned in a way that allows quick separation from the mains.
!
Warning: Never use the device in explosive atmospheres or in explosive mixtures of anaesthetics with oxygen or laughing gas.
Conditions for Transport and Storage The following transport and storage conditions must be met: temperature: -40°C to +70°C (-40°F to +158°F) ambient pressure: 500 hPa to 1060 hPa relative humidity: 10% to 100% (no condensation)
During transport and storage the devices must be kept dry. Don’t turn them upside down or place them on the side. The devices are fragile. Don’t use hand hooks for transport. Pile up a maximum of 2 boxes.
The symbols attached to the packaging have the following meaning: Upside Fragile Keep dry No hand hooks 2 boxes only
1-10
MDTdig e, Rev. 2
Compumedics Germany GmbH
Instructions on Disposal The device and the accessories have to be disposed of according to national laws and guidelines as electro and electronical devices separate from normal waste. Warning: Accessories that come into contact with patients have to be decontaminated first, or they have to be disposed of as potentially infectious hazardous waste. For more detailed information on how to dispose of your device correctly contact your local authorities or your distributor.
MDTdig e, Rev. 2
!
1-11
Compumedics Germany GmbH
2. The Design This chapter describes the basic unit and the system components that comprise the MultiDop® T digital. Furthermore you can find information on how to set up the system. Only a few connections have to be made. multi-touch display stand-by key green: running blue: stand-by hard disk activity CO2 activity removable Bluetooth keyboard
Figure 2-1: System MultiDop® T digital
probe holder
probe sockets
cover of CO2 connectors (option)
Figure 2-2: Compartment for remote control remote control in its compartment
Warning: The multi-touch TFT colour display (17.3” = 43.9 cm, full HD 1920x1080) with tablet function is highly sensitive and should be handled with greatest care. Never apply too much on the screen. Keep it away from intense, direct sunlight as this will destroy or damage the display.
MDTdig e, Rev. 2
! 2-1