CooperSurgical Inc
ALLY Uterine Positioning System
ALLY Uterine Positioning System Instructions for Use Rev A Oct 2017
Instructions for Use
120 Pages
Preview
Page 1
TABLE OF CONTENTS (ENGLISH) SECTION 1
Introduction...2
SECTION 2
Indications for Use ... 2
SECTION 3
Contraindications ... 2
SECTION 4
Warnings and Precautions ... 2 Warnings...2 Precautions...3
SECTION 5
Device Description ...4
SECTION 6
Directions for Use ... 6
SECTION 7
Guidance and Manufacturer’s Declaration – Electromagnetic Emissions ...10
SECTION 8
Guidance and Manufacturer’s Declaration – Electromagnetic Immunity (for all Medical Equipment and Medical Equipment Systems) ... 11
SECTION 9
Guidance and Manufacturer’s Declaration – Electromagnetic Immunity (for all Medical Equipment and Medical Equipment Systems that are not Life-Supporting) ... 12
SECTION 10
Recommended Separation Distances between Portable and Mobile RF Communications Equipment and ALLY UPS™ ... 13
SECTION 11
Disposal ... 13
SECTION 12
Accessories ... 13
SECTION 13
Warranty ... 13
SECTION 14
Specifications ...14
SECTION 15
Explanation of Symbols ... 15
1
ALLY Uterine Positioning System (ENGLISH) ®
SECTION 1 INTRODUCTION Read all information carefully. Failure to properly follow all instructions, including those supplied with the CooperSurgical manipulator handles and adapters may lead to injury and result in improper functioning of the device. CAUTION: U.S. Federal law restricts this device to sale by or on the order of a physician.
SECTION 2 INDICATIONS FOR USE The ALLY Uterine Positioning System® (ALLY UPS®) is intended to assist the surgical staff in mounting, positioning and holding uterine manipulators during gynecological laparoscopic surgical procedures. It is intended for use by trained operating room personnel in an operating room environment.
SECTION 3 CONTRAINDICATIONS CooperSurgical manipulator handles should not be used in patients who are pregnant or who are suspected of being pregnant, planned gamete intrafallopian transfer procedures, in patients who have an IUD in place, in patients with suspected pelvic infections and in cases where the surgeon deems it inadvisable or finds it difficult to insert the silicone tip into the cervix or uterus.
SECTION 4 WARNINGS AND PRECAUTIONS WARNINGS • To avoid risk of electric shock, this equipment must only be connected to a supply mains with protective earth. • The ALLY UPS is only intended for use with CooperSurgical’s family of uterine manipulators. For a complete list of the manipulators compatible with the ALLY UPS, please contact CooperSurgical Customer Service. Use of this device in any configuration other than specified is not recommended, and could lead to injury and improper functioning of each device • Flammable Anesthetics: This device is not suitable for use in the presence of a flammable anesthetic mixture with air, or in the presence of a flammable anesthetic mixture with oxygen or nitrous oxide. • Read all instructions carefully. Failure to properly follow instructions may cause improper functioning of the device. • - Use only the rail clamp supplied with the ALLY UPS. - Do not install rail clamp across different rail sections. • Surgical procedures requiring vaginal instrumentation are not sterile. Conventional Operating Room procedures for maintaining sterility must be observed when the ALLY UPS is in use. • DO NOT USE EXCESSIVE FORCE. If adequate range of motion is not obtained, reposition the ALLY UPS (reference section 6.2 on proper positioning). If problem persists, discontinue use. •
DO NOT store the device in direct sunlight, at high temperatures or high humidity.
• • • • • •
DO NOT store this device in the shipping box. ALWAYS position the patient and the operating room table prior to attaching the ALLY UPS to the manipulator. ALWAYS have patient under general endotracheal anesthesia when ALLY UPS is attached to the manipulator. ALWAYS use caution when attaching and detaching the manipulator from the ALLY UPS. DO NOT move the foot end of the Operating Room table while the ALLY UPS is attached to the table. ALWAYS follow all instructions and recommendations discussed in the Uterine Manipulator Directions for Use. ALWAYS handle the ALLY UPS with care. Avoid mechanical shock or stress that can cause damage to the device. DO NOT carry the ALLY UPS using anything other than the blue handle and blue fixed arm area. The foot pedal cover should be used to lift, carry, or reposition the foot pedal.
• •
2
ALLY Uterine Positioning System (ENGLISH) ®
PRECAUTIONS • Always ensure that the ALLY UPS is tightly and securely attached to the table prior to the start of the surgical procedure. Improper or loose mounting of the system can lead to unintended movement and may lead to injury. •
Users should securely hold the manipulator handle before the foot pedal is depressed to avoid inadvertent movement of the arm.
•
Inspect the adapter drape prior to use for damage. Ensure that the packaging of the adapter drape has not been breached. Verify the expiration date.
•
Operating room personnel should take care not to contaminate the draped ALLY UPS during the remaining patient preparation steps.
•
DO NOT attach the manipulator to the ALLY UPS arm until after the da Vinci Surgical System patient side cart is in position and the brakes are set.
•
DO NOT attach the manipulator to the ALLY UPS arm until after the patient is in the final position.
•
Users should check that the adapter is holding the manipulator securely without any extraneous movement prior to operative use. If the manipulator does not securely attach to the manipulator adapter discontinue use immediately.
•
The ALLY UPS MUST be removed from the table PRIOR to the foot end/pins being returned to the horizontal position.
•
Damage will result if the flexible arm is cleaned with bleach products.
•
Damage may result if the rail clamp is cleaned with bleach.
•
Care should be taken during storage and transportation to avoid drops, falls, mechanical stress, and mechanical shock to the device.
ATTENTION Emergency Power Removal Advice Do not position the ALLY UPS so that it is difficult to remove the disconnect device (plug). If there is an immediate need to remove power from the ALLY UPS, disconnecting the power cord from the ALLY UPS unit will stop it from functioning. 4.1 REQUIREMENTS APPLICABLE TO THE ALLY UTERINE POSITIONING SYSTEM The ALLY UPS needs special precautions regarding EMC and needs to be installed and put into service according to EMC information provided in the tables in Sections 8 through 11. Portable and mobile RF communications equipment can affect the ALLY UPS.
3
ALLY Uterine Positioning System (ENGLISH) ®
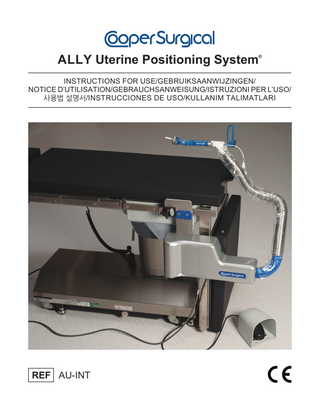
SECTION 5 DEVICE DESCRIPTION The ALLY UPS attaches to the operating room table and will enable the bed-side assistant to readily mount, hold, and position the manipulator during laparoscopic surgical procedures. The ALLY UPS enables access and provides the ability to maneuver and maintain the manipulator in a desired position. The ALLY UPS consists of the ALLY UPS and the manipulator adapter with built-in sterile drape (sold separately) known as the adapter drape. Figure 1 shows the system followed by descriptions of its various parts. Manipulator Adapter
NON-STERILE Must drape prior to use as shown
Sterile Drape
ALLY UPS Rail Clamp
Flexible Arm
ALLY UPS Handle
Power Switch Fixed Arm
Main Unit
Power Cord
Foot Pedal Cord
Elbow Foot Pedal Cover Foot Pedal
Figure 1: ALLY Uterine Positioning System (ALLY UPS)
4
ALLY Uterine Positioning System (ENGLISH) ®
There are three main components to the ALLY UPS System:
(1) MAIN UNIT ASSEMBLY Main Unit • The main unit houses the controls of the ALLY Uterine Positioning System. Power Switch • The power switch must be ON (green light) in order for the system to work. Foot Pedal • Controls the locking and unlocking of the arm. • Depressing the foot pedal releases the arm to allow for maneuvering the manipulator. • Releasing the foot pedal locks the arm and holds the manipulator in the desired position. Flexible Arm / Fixed Arm • Provides desired range-of-motion for positioning the manipulator. • Holds the manipulator in the desired position. • Has a mating tip to accommodate engagement with adapter. ALLY UPS Blue Handle • This is what is held onto (as well as the blue elbow) when installing or removing the ALLY UPS from the rail. Refer to Section 7.1 for more information. Power Cord • Detachable cord that acts as a disconnect device – supplies power to the main unit and foot pedal.
(2) ALLY UPS RAIL CLAMP • •
Used to mount the ALLY UPS to the patient’s right side rail of standard operating room tables. Secure by tightening the knob.
(3) ADAPTER DRAPE
(sold separately) (Part Numbers AU-AD and AU-AD-DLNTR) • • • • • • •
The adapter drape connects the flexible arm to CooperSurgical manipulators. This component is a single-use disposable and comes with drape attached. One end of the adapter attaches to the flexible arm via pinch clips. The other end of the adapter securely attaches to the manipulator using the latching mechanism. The ALLY UPS’s arm must be completely draped to ensure that the sterile field is not compromised during the surgical procedure. The AU-AD to be used with RUMI and Advincula Arch. The AU-AD-DLNTR to be used with the Advincula Delineator
AU-AD
AU-AD-DLNTR
5
ALLY Uterine Positioning System (ENGLISH) ®
SECTION 6 DIRECTIONS FOR USE
!
WARNING: Read all instructions carefully. Failure to properly follow instructions may cause improper functioning of the device.
6.1 INSPECTION PRIOR TO USE Prior to each use, all components should be inspected for damage or irregularities. The device should not be used if any damage or irregularity is observed. The user should contact CooperSurgical Customer Service if any damage or irregularities are noticed. The sequence of operations listed below is recommended to ensure safe and effective functioning of the device. 6.2 MOUNTING THE ALLY UPS 1. Position patient on operating room table in dorsal lithotomy position with legs in stirrups. Refer to leg stirrup manufacturer’s instructions for proper use and positioning. Ensure patient is placed such that the buttocks are as close to edge as possible. 2. Lower or remove foot end of the operating room table. 3. Attach the ALLY UPS to the operating room table by placing the rail clamp on the patient’s right side near the stirrup. Lower the mounting tab of the ALLY UPS into the rail clamp.
Approximate location of introitus
4. Tighten the knob by hand until secure. Check security of hold by trying to slide off the rail. Figure 2a shows how to install the rail clamp over the cutouts in the rail. Figure 2b shows the “final” position of the clamp once it is on the rail.
For optimum position, locate the top corner of ALLY UPS within the area between the introitus and the end of the pad.
Figure 2a: Installing the rail clamp
Figure 2b: Move the clamp to the right away from the slots in the rail
Rail clamp #1 for the ALLY UPS
(A)
Rail clamp #2 for the stirrup
NOTE: Leave a minimum gap of 3” (7.6 cm) between rail clamps for the mounting bar.
Figure 2c: Installing the main unit into the ALLY UPS rail clamp
6
ALLY Uterine Positioning System (ENGLISH) ®
!
WARNING: • Use only the rail clamp supplied with the ALLY UPS. • Do not install rail clamp across different rail sections.
!
CAUTION: Always ensure that the ALLY UPS is tightly and securely attached to the table prior to the start of the surgical procedure. Improper or loose mounting of the system can lead to unintended movement and may lead to injury.
ALLY UPS Rail Clamp
Stirrup Rail Clamp
Stirrup
ALLY UPS Main Unit
Figure 2d: ALLY UPS in position in the ALLY UPS rail clamp (to the left of the stirrup clamp)
5. Attach the power cord to the main unit (making sure it is firmly in place) and attach it to the nearest outlet. If an extension cord is needed, make sure it is a medical grade cord suitable to withstand 125VAC/10 amps. NOTE: Position foot pedal within reach of operating room personnel actuating the ALLY UPS. It is recommended to position the ALLY UPS foot pedal separately from other foot pedals.
!
CAUTION: Users should securely hold the manipulator handle before the foot pedal is depressed to avoid inadvertent movement of the arm.
6. Turn the power on and verify that the green light indicator is lit. Press and hold the foot pedal once. Release to initialize the ALLY UPS. The ALLY UPS arm can be moved by grasping the flexible arm and depressing the foot pedal. Releasing the foot pedal locks the ALLY UPS in place, holding the arm in a static position. 7. Position the ALLY UPS flexible arm out of the way prior to patient preparation. It is recommended to place a chuck over the arm while prepping the patient.
7
ALLY Uterine Positioning System (ENGLISH) ®
6.3 ATTACHING THE ADAPTER DRAPE
! !
WARNING: Surgical procedures requiring vaginal instrumentation are not sterile. Conventional Operating Room procedures for maintaining sterility must be observed when the ALLY UPS is in use. CAUTION: Inspect the adapter drape prior to use for damage. Ensure that the packaging of the adapter drape has not been breached. Verify the expiration date.
NOTE: It is recommended to have spare adapter drapes ready at the start of a case. 1. Once patient prep is complete, and prior to draping the patient, it is time to connect the adapter drape to the ALLY UPS. 2. The sterile personnel should connect the adapter drape. Align the pinch clips as shown and press them onto the end of the flexible arm. Make sure the drape snaps fully into place. Slide the attached drape around the elbow of the ALLY UPS until you get to the main unit.
Figure 3a: Holding the sterile drape
Figure 3b: Final position of the drape in the connector
!
Figure 3c: Fully extended drape
CAUTION: Operating room personnel should take care not to contaminate the draped ALLY UPS flexible arm during the remaining patient preparation steps.
3. It is recommended to drape the patient’s legs at this step in the process. NOTE: Continue with necessary patient preparation steps. 6.4 WHEN AN INTUITIVE SURGICAL DA VINCI® SYSTEM PATIENT SIDE CART IS IN USE 1. 2. 3. 4.
Place the ALLY UPS arm in approximate position for attachment near the proximal end of the manipulator. Position the patient side cart. Insert the manipulator, and then attach it to the ALLY UPS per the adapter’s instructions. Continue with the docking of the da Vinci Surgical System arms.
!
CAUTION: DO NOT attach the manipulator to the ALLY UPS arm until after the da Vinci Surgical System patient side cart is in position and the brakes are set. 8
ALLY Uterine Positioning System (ENGLISH) ®
6.5 INTRA-OPERATIVE USE 1. 2. 3. 4.
Grasp the handle of the manipulator. Depressing the foot pedal allows for dynamic manipulation of the flexible arm. Maneuver to the desired position. Releasing the foot pedal locks the ALLY UPS in place, holding the manipulator in a static position.
!
WARNING: DO NOT USE EXCESSIVE FORCE. If adequate range of motion is not obtained, reposition the ALLY UPS (reference section 6.2 on proper positioning). If problem persists, discontinue use.
!
CAUTION: DO NOT attach the manipulator to the ALLY UPS arm until after the patient is in the final position.
!
CAUTION: Users should check that the adapter is holding the manipulator securely without any extraneous movement prior to operative use. If the manipulator does not securely attach to the manipulator adapter discontinue use immediately.
6.6 REMOVING THE ALLY UPS 1. Detach the manipulator from the adapter drape by opening the latch per the Instructions for Use provided with the adapter. Detach the manipulator from the adapter drape prior to extracting the uterus during a hysterectomy procedure. 2. If used, undock the da Vinci Surgical System and move patient side cart away from the operating room table. 3. Detach the adapter by squeezing the pinch clips together. 4. Remove the used sterile drape and dispose of properly. NOTE: Refer to the adapter drape’s Instructions for Use for complete instructions.
!
CAUTION: The ALLY UPS MUST be removed from the table PRIOR to the foot end/pins being returned to the horizontal position.
6.7 POWERING DOWN THE ALLY UPS 1. Press the foot pedal while powering down so that the arm remains in its flexible state when dismounting and storing. 2. Turn off the power switch and unplug from the wall outlet. 3. Fully loosen the knob on the operating room table rail camp until the ALLY UPS is no longer firmly secured to the operating room table. The ALLY UPS can be lifted off the rail clamp. Figure 4 shows how to remove the ALLY UPS main unit from the rail. 4. Remove the rail clamp and store it with the ALLY UPS.
Figure 4: Removing the ALLY UPS main unit from the rail
9
ALLY Uterine Positioning System (ENGLISH) ®
6.8 CLEANING AND STORAGE 1. The ALLY UPS system, including the main unit, fixed arm, flexible arm, handle and foot pedal should be cleaned after each use. Only use Isopropyl Alcohol 70 percent (70%) or higher. Wipe all surfaces free of debris. Do not spray flexible arm. Ensure flexible arm is thoroughly dry before powering on. 2. After each use clean the rail clamp; clean and disinfect using a quaternary ammonium disinfecting/ cleaning solution, read the cleaning product’s directions and follow the instructions on the label for recommendation for achieving low-level disinfection; use caution around the knob where fluid migration may occur. Wipe device with a clean, dry cloth; make certain the product is dry prior to reinstalling to avoid damage.
!
CAUTION: Damage will result if the flexible arm is cleaned with bleach products.
!
CAUTION: Damage may result if the rail clamp is cleaned with bleach.
!
CAUTION: Care should be taken during storage and transportation to avoid drops, falls, mechanical stress, and mechanical shock to the device.
3. The foot pedal cover should be used to lift, carry or reposition the foot pedal. 4. Store this device in a clean, dry and well-ventilated environment.
!
WARNING: DO NOT store the device in direct sunlight, at high temperatures or high humidity.
!
WARNING: DO NOT store this device in the shipping box.
SECTION 7 GUIDANCE AND MANUFACTURER’S DECLARATION – ELECTROMAGNETIC EMISSIONS (for all Medical Equipment and Medical Equipment Systems) The ALLY UPS is intended for use in the electromagnetic environment specified below. The customer or the user of the ALLY UPS should assure that it is used in such an environment. EMISSIONS TEST
COMPLIANCE
ELECTROMAGNETIC ENVIRONMENT GUIDANCE
RF emissions CISPR 11 Group 1
The ALLY UPS uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment.
RF emissions CISPR 11 Class A Harmonic emissions IEC 61000-3-2 Class A Voltage fluctuations/flicker emissions Complies IEC 61000-3-3
The ALLY UPS is suitable for use in all establishments other than domestic and those directly connected to the public low voltage power supply network that supplies buildings used for domestic purposes.
10
ALLY Uterine Positioning System (ENGLISH) ®
SECTION 8 GUIDANCE AND MANUFACTURER’S DECLARATION – ELECTROMAGNETIC IMMUNITY (for all Medical Equipment and Medical Equipment Systems) The ALLY UPS is intended for use in the electromagnetic environment specified below. The customer or the user of the ALLY UPS should assure that it is used in such an environment. IMMUNITY TEST IEC 60601 TEST LEVEL COMPLIANCE LEVEL
ELECTROMAGNETIC ENVIRONMENT GUIDANCE
Electrostatic discharge (ESD) ±6 kV contact ±6 kV contact IEC 61000-4-2 ±8 kV air ±8 kV air
Floors should be wood, concrete, or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%.
Electrical fast transient/burst IEC 61000-4-4
Mains power quality should be that of a typical commercial or hospital environment.
±2 kV for power ±2 kV for power supply lines supply lines ±1 kV for input/output lines ±1 kV for input/output lines
Surge ±1 kV line(s) to line(s) ±1 kV line(s) to line(s) Mains power quality should be that IEC 61000-4-5 ±2 kV line(s) to earth ±2 kV line(s) to earth of a typical commercial or hospital environment. Voltage dips, short interruptions and voltage variations on power supply input lines IEC 61000-4-11
<5 % UT (>95 % dip in UT) for 0.5 cycle
<5 % UT (>95 % dip in UT) for 0.5 cycle
40 % UT 40 % UT (60 % dip in UT) (60 % dip in UT) for 5 cycles for 5 cycles 70 % UT 70 % UT (30 % dip in UT) (30 % dip in UT) for 25 cycles for 25 cycles <5 % UT (>95 % dip in UT) for 5 s
Mains power quality should be that of a typical commercial or hospital environment. If the user of the ALLY UPS requires continued operation during power mains interruptions, it is recommended that the ALLY UPS be powered from an uninterruptible power supply or a battery.
<5 % UT (>95 % dip in UT) for 5 s
Power frequency (50/60 Hz) 3 A/m 3 A/m magnetic field IEC 61000-4-8 NOTE: UT is the a.c. mains voltage prior to application of the test level.
11
Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment.
ALLY Uterine Positioning System (ENGLISH) ®
SECTION 9 GUIDANCE AND MANUFACTURER’S DECLARATION – ELECTROMAGNETIC IMMUNITY (for all Medical Equipment and Medical Equipment Systems that are not Life-Supporting) The ALLY UPS is intended for use in the electromagnetic environment specified below. The customer or the user of the ALLY UPS should assure that it is used in such an environment. ELECTROMAGNETIC IMMUNITY TEST IEC 60601 TEST LEVEL COMPLIANCE LEVEL ENVIRONMENT GUIDANCE Portable and mobile RF communications equipment should be used no closer to any part of the Ally UPS, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter. Recommended separation distance Conducted RF IEC 61000-4-6
3 Vrms 3 Vrms 150 kHz to 80 MHz
d = 1.17 √P
Radiated RF 3 V/m 3 V/m d = 1.17 √P 80 MHz to 800 MHz IEC 61000-4-3 ±2 kV line(s) to earth d = 2.33 √P 800 MHz to 2.5 GHz
where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in meters (m).
Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey,a should be less than the compliance level in each frequency range.b
Interference may occur in the vicinity of equipment marked with the following symbol:
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies. NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast, and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the Ally UPS is used exceeds the applicable RF compliance level above, the ALLY UPS should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the ALLY UPS.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
12
ALLY Uterine Positioning System (ENGLISH) ®
SECTION 10 RECOMMENDED SEPARATION DISTANCES BETWEEN PORTABLE AND MOBILE RF COMMUNICATIONS EQUIPMENT AND ALLY UPS (for all Medical Equipment and Medical Equipment Systems that are not Life Supporting) The ALLY UPS is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the ALLY UPS can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the ALLY UPS as recommended below, according to the maximum output power of the communications equipment. RATED MAXIMUM OUTPUT POWER OF TRANSMITTER IN WATTS
SEPARATION DISTANCE ACCORDING TO FREQUENCY OF TRANSMITTER (Meters) [Notes 1 and 2] 150 kHz to 80 MHz d = 1.17 √P
80 MHz to 800 MHz d = 1.17 √P
800 MHz to 2,5 GHz d = 2.33 √P
0.01 0.1
0.117 0.37
0.117 0.37
0.233 0.737
1
1.17
1.17
2.33
10
3.7
3.7
7.37
100
11.7
11.7
23.3
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be determined using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
SECTION 11 DISPOSAL Dispose of in accordance with all applicable Federal, State, and Local Medical/Hazardous waste practices.
SECTION 12 ACCESSORIES The following accessories are available from CooperSurgical. DESCRIPTION
PART NUMBER
Adapter Drape
AU-AD
Adapter Drape
AU-AD-DLNTR
CART
AU-CART
One of the AU Accessory Kits has been supplied with the ALLY UPS: PART NUMBER
CONTENTS
AU-KITF AU-KITG AU-KITH AU-KITM AU-KITI
Power Cord IEC Type F; Rail Clamp UK/EURO Power Cord IEC Type G; Rail Clamp UK/EURO Power Cord IEC Type H; Rail Clamp UK/EURO Power Cord IEC Type M; Rail Clamp UK/EURO Power Cord IEC Type I; Rail Clamp DENYER
SECTION 13 WARRANTY CooperSurgical warrants that the ALLY UPS will be free from defects in materials and workmanship for one year from the date of purchase. If CooperSurgical, Inc. determines that the ALLY UPS fails to perform within that one year, as the sole remedy for that failure to perform, at CooperSurgical’s discretion, we will repair or replace the ALLY UPS free of charge. 13
ALLY Uterine Positioning System (ENGLISH) ®
SECTION 14 SPECIFICATIONS Dimensions (H x W x D):... 14.7 inches x 21.5 inches x 17.0 inches (37.4 cm x 54.6 cm x 43.2 cm) Depth of the Main Unit:... 4.5 inches (11.4 cm) Weight:... approximately 28 pounds (12.7 kg) Power Requirement Main Supply:... 100-240VAC, 50/60Hz Rating:... 1.6 Amps Fuse:... 250 V / 2.0 A, Type T, Slow Blow Classification: ... I IP Rating: Main Unit:... IP31 Foot Pedal:... IPX6 Average Duty Cycle Rating:... 5 seconds ON, 300 seconds OFF No customer-replaceable components inside the main unit assembly Environmental Conditions Operational: Temperature: ... 68 °F to 90 °F (20 °C to 32 °C) Humidity: ... 20% RH to 60% RH Air Pressure:... 21 inHg to 31 inHg (70 kPa to 106 kPa) Shipping and Storage: Temperature:... -40 °F to 158 °F (-40 °C to 70 °C) Humidity:... 10% RH to 100% RH Air Pressure:... 15 inHg to 31 inHg (50 kPa to 106 kPa)
14.7” (37.4 cm)
17.0” (43.2 cm)
21.5” (54.6 cm)
Figure 5: Front and side views of main unit and arm
4.5” (11.4
21.5” (54.6 cm)
Figure 6: Top view of main unit
14
ALLY Uterine Positioning System (ENGLISH) ®
SECTION 15 EXPLANATION OF SYMBOLS Reorder number Serial number
Rx Only
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
UL Rating/Approved MEDICAL EQUIPMENT WITH RESPECT TO ELECTRICAL SHOCK, FIRE AND MECHANICAL HAZARD ONLY IN ACCORDANCE WITH AAMI/ANSI ES 60601-1 AND CAN/CSA C22.2 NO. 60601.1:08
6D33 Consult instructions for use
! LATEX
Caution
Not made with natural rubber latex
Authorized Representative in the European Community.
Manufacturer
ALLY Uterine Positioning System® is a registered trademark of CooperSurgical, Inc. da Vinci® is a registered trademark of Intuitive Surgical, Inc. © 2017 CooperSurgical, Inc. All rights reserved. Made in the USA
15