Datex-Ohmeda
Datex-Ohmeda S5 Monitor Series
S5 Anesthesia and Critical Care Monitor Technical Reference Manual Oct 2003
Technical Reference Manual
84 Pages
Preview
Page 1
Datex-Ohmeda S/5™ Anesthesia Monitor and S/5™ Critical Care Monitor Technical Reference Manual
All specifications are subject to change without notice. CAUTION: U.S. Federal law restricts this device to sale by or on the order of a licensed medical practitioner. Outside the USA, check local laws for any restriction that may apply. Document No. 8005796 October 2003 Datex-Ohmeda, Inc. P.O. Box 7550, Madison WI 53707-7550, USA Tel. 1-608-221-1551 Fax 1-608-222-9147 mailto:[email protected] www.us.datex-ohmeda.com
Datex-Ohmeda Division, Instrumentarium Corp. P.O. Box 900, FIN-00031 DATEX-OHMEDA, FINLAND Tel. +358 10 394 11 Fax +358 9 146 3310 www.datex-ohmeda.com Instrumentarium Corp. All rights reserved.
S/5 Anesthesia Monitor Intended purpose (Indications for use) The Datex-Ohmeda S/5 Anesthesia Monitor with L-ANE03 or L-ANE03A software is intended for multiparameter patient monitoring with optional patient care documentation. The S/5 Anesthesia Monitor with L-ANE03 and L-ANE03A software is indicated for monitoring of hemodynamic (including arrhythmia and ST-segment analysis), respiratory, ventilatory, gastrointestinal/regional perfusion, Bispectral index (BIS), Entropy (State Entropy and Response Entropy) and neurophysiological status of all hospital patients. The S/5 Anesthesia Monitor with L-ANE03 and L-ANE03A software when using BIS is for monitoring the state of the brain by data acquisition and processing of electroencephalograph signals and may be used as an aid in monitoring the effects of certain anesthetic agents. The S/5 Anesthesia Monitor with L-ANE03 and L-ANE03A software is also indicated for documenting patient care related information. The S/5 Anesthesia Monitor with L-ANE03 and L-ANE03A software is indicated for use by qualified medical personnel only. S/5 Critical Care Monitor Intended purpose (Indications for use) The Datex-Ohmeda S/5 Critical Care Monitor with L-ICU03 or L-ICU03A software is intended for multiparameter patient monitoring. The S/5 Critical Care Monitor with L-ICU03 and L-ICU03A software is indicated for monitoring of hemodynamic (including arrhythmia and ST-segment analysis), respiratory, ventilatory, gastrointestinal/regional perfusion, Bispectral index (BIS), and neurophysiological status of all hospital patients. The S/5 Critical Care Monitor with L-ICU03 and L-ICU03A software when using BIS is for monitoring the state of the brain by data acquisition and processing of electroencephalograph signals and may be used as an aid in monitoring the effects of certain anesthetic agents. The S/5 Critical Care Monitor with L-ICU03 and L-ICU03A software is indicated for use by qualified medical personnel only. Classifications In accordance with IEC 60601-1 CLASS I EQUIPMENT - the type of protection against electric shock. TYPE BF or CF equipment. The degree of protection against electric shock is indicated by a symbol on each parameter module. EQUIPMENT not suitable for use in the presence of a FLAMMABLE ANESTHETIC MIXTURE WITH AIR OR WITH OXYGEN OR NITROUS OXIDE. CONTINUOUS OPERATION according to the mode of operation. In accordance with IEC 60529 Degree of protection against the harmful ingress of water as detailed in the IEC 60529: IPX0 In accordance with EU Medical Device Directive The Datex-Ohmeda S/5 Anesthesia Monitor and S/5 Critical Care Monitor are classified as IIb.
Responsibility of the manufacturer Datex-Ohmeda Division, Instrumentarium Corp. is responsible for the safety, reliability and performance of the equipment only if: −
assembly, operations, extensions, readjustments, modifications, service and repairs are carried out by personnel authorized by Datex-Ohmeda.
−
the electrical installation complies with appropriate requirements.
−
the equipment is used in accordance with the User’s Guide.
−
the system is serviced, maintained and used in accordance with the Technical Reference Manual.
Trademarks Datex®, Ohmeda®, and other trademarks S/5, Entropy, D-lite, D-lite+, Pedi-lite, Pedi-lite+, Mini D-fend, Dfend, D-fend+, TruTrak®+, OxyTip+, MemCard, ComWheel, ComBar, EarSat, FingerSat, FlexSat, PatientO2, Patient Spirometry and Tonometrics are property of Instrumentarium Corp. or its subsidiaries. All other product and company names are property of their respective owners.
Copyright © Copyright Instrumentarium Corp. 2002 All rights reserved. All specifications subject to change without notice.
Master table of contents
Datex-Ohmeda S/5TM Anesthesia Monitor and Critical Care Monitor Technical Reference Manual, 8005796 PART I, General Service Guide Document No.
Updated
Updated
Description
8005796
Introduction, System description, Installation, Interfacing, Functional check, General troubleshooting
1
8005673
Planned Maintenance Instructions
2
PART II, Product Service Guide 8-Module Frame, F-CU8
1
CPU Board, B-CPU5 Software Licences, L-ANE03, L-ANE03A, L-ICU03, L-ICU03A
2
UPINET Board, B-UPI4NET
3
Video Displays, LCD Displays, Plasma Display, Display Controllers
4
8001589 -4
Command Bars, K-ANEB, K-ICUB
5
8001005 - 5
Airway Modules, G-AiOV, G-AiO, G-AOV, G-AO, Gas Interface Board, B-GAS
6
8001006 -3
Interface Board, B-INT
7
8001007 -3
Extension Frame, F-EXT4, Extension Module, M-EXT
8
8004317-2 8005795
8004319-2 8005675
For S/5TM modules specific information see the Technical Reference Manual of the S/5 Modules
Document No. 8005796
S/5™ Anesthesia Monitor and Critical Care Monitor
Document No. 8005796
Table of contents
TABLE OF CONTENTS S/5™ Anesthesia Monitor and S/5™ Critical Care Monitor TABLE OF CONTENTS
i
TABLE OF FIGURES
iv
1
1
INTRODUCTION 1.1
Notes to the reader... 2 1.1.1 Related documentation... 2 1.1.2 Conventions used ... 3 1.2 Symbols ... 4 1.2.1 Symbols on transport packaging... 4 1.2.2 Symbols on equipment ... 4 1.3 Safety... 7 1.3.1 Classification... 7 1.3.2 Responsibility of the manufacturer... 7 1.3.3 Safety precautions... 7
2
SYSTEM DESCRIPTION 2.1 2.2 2.3 2.4 2.5 2.6
3
11
Introduction ... 11 Bus structure ... 11 Distributed processing... 11 Module communication... 12 Software loading ... 13 Parameter modules... 13
SYSTEM INSTALLATION
14
3.1 3.2 3.3
Unpacking instructions ... 14 Choosing location ... 14 Central Unit; S/5 8-Module Frame, F-CU8 ... 15 3.3.1 Connecting to mains ... 15 3.3.2 Connecting to Datex-Ohmeda Network... 15 3.3.3 Inserting the parameter modules ...16 3.3.4 Positioning of PC boards... 17 3.3.5 Replacing PC Boards... 18 3.3.6 Performing factory reset ... 18 3.4 Displays... 19 3.4.1 Main displays ... 19 3.4.2 Secondary displays... 19 3.4.3 3rd display ... 19 3.4.4 S/5 Video Display, D-VMC15 ...19 3.4.5 S/5 LCD Display, D-LCC15... 20 3.4.6 15” Video Display, D-VNC15 ... 21 3.4.7 17” Video Display, D-VHC17, revision 00-01 ... 22 3.4.8 17” Video Display, D-VHC17, revision 02-03 ... 22 3.4.9 10” LCD Display, D-LCC10A/W ... 22 3.4.10 S/5 LCD Display, D-LCC17 ... 23 3.4.11 S/5 LCD Display, D-LCC19 ... 23 3.4.12 21" Display Monitor Unit, D-VSC21... 25 3.4.13 43" Plasma Display, D-MMP43 ... 25 3.5 Display controller boards ... 26 3.5.1 Jumper settings ... 26 i Document No. 8005796
Datex-Ohmeda S/5 Anesthesia Monitor and S/5 Critical Care Monitor 3.5.2 Resolution selection for B-DISPX ... 28 S/5 Remote Controller, K-REMCO... 29 S/5 Airway Modules, G-XXXX ... 29 3.7.1 Connection to Central Unit ... 29 3.7.2 Sample gas exhaust... 29 3.7.3 Returning sample gas to patient circuit ... 30 3.8 Record Keeping Keyboard for Anesthesia, K-ARKB... 30 3.8.1 Connection to Central Unit ... 30 3.8.2 Connection to LCD Display, D-LCC10A/W... 31 3.9 ARK Barcode Reader, N-SCAN (optional)... 31 3.9.1 Connection to Central Unit/LCD Display, D-LCC10A/W ... 31 3.10 S/5 Extension Frame, F-EXT4 ... 32 3.10.1 Mounting of Extension Frame, F-EXT4 ... 33 3.10.2 Connection to Central Unit ... 33 3.10.3 Inserting parameter modules ... 33 3.10.4 Troubleshooting... 34 3.6 3.7
4
INTERFACING
35
4.1
Interfacing external monitors via Interface Module, M-INT, or Interface Board, B-INT ... 35 4.1.1 Connecting interface connector cables to Interface Board, B-INT... 36 4.1.2 Connection to external Datex-Ohmeda monitors... 37 4.1.3 Connection to Critikon Dinamap 1846SX, Abbott Oximetrix 3 and Baxter Explorer ... 37 4.1.4 Connection to Baxter Vigilance ... 37 4.1.5 Connection to Nellcor N-100 and N-1000... 38 4.1.6 Connection to Nellcor N-200... 38 4.2 Interfacing external bedside devices via S/5 Device Interfacing Solutions, N-DISxxx... 38 4.2.1 Interfaced devices and parameters... 39 4.2.2 Device Interfacing Solution components ... 41 4.2.3 Connections ... 41 4.2.4 Mounting... 41 4.2.5 Selecting the external device ... 43 4.2.6 Selecting the parameter data source ... 43 4.3 Interfacing Datex-Ohmeda Anesthesia Delivery Unit... 43 4.3.1 Interconnection ... 43 4.3.2 Setting interfacing parameters on the S/5 Anesthesia Delivery Unit ... 43 4.3.3 Setting interfacing parameters on the S/5 Anesthesia Monitor... 44 4.4 Interfacing Dräger Cicero, Cato, Julian and Narkomed 2C (by NAD)... 45 4.4.1 Interconnection ... 45 4.4.2 Setting communication parameters... 45 4.4.3 Setting interfacing parameters on the S/5 Anesthesia Monitor or S/5 Critical Care Monitor... 45 4.5 Interfacing printer... 46 4.5.1 Connection to HP LaserJet 4P printer ... 46 4.5.2 Connection to Epson EPL-5200 printer ... 47 4.5.3 Connection to Epson EPL-5500 printer ... 48 4.6 Interfacing computer ... 49 4.7 UPI4 and UPI4NET Board Output Signals... 49 4.7.1 Digital outputs... 50 4.7.2 Analog outputs ... 50 4.8 S/5 Pressure Temp Module, M-PT, output signals ... 51 4.8.1 Analog outputs ... 51
5
FUNCTIONAL CHECK 5.1 5.2
52
Recommended tools ... 52 Visual inspection... 53
ii Document No. 8005796
Table of contents 5.3
6
Functional inspection ... 53 5.3.1 General ... 53 5.3.2 Display(s) ... 54 5.3.3 Keyboard(s)... 54 5.3.4 8-Module Frame, F-CU8... 54 5.3.5 Extension Frame, F-EXT4 ... 54 5.3.6 Airway Module, G-XXXX... 55 5.3.7 Compact Airway Module, M-CXXXXX... 55 5.3.8 Single width Airway Module, M-miniC... 56 5.3.9 Tonometry Module, M-TONO...56 5.3.10 Hemodynamic Modules ... 56 5.3.11 Pressure/Pressure Temp Modules, M-P/-PT ... 57 5.3.12 Dual pressure Module, M-PP ... 58 5.3.13 Cardiac Output Modules, M-COP/-COPSv ... 58 5.3.14 NIBP module, M-NIBP...58 5.3.15 Nellcor Compatible Saturation module, M-NSAT... 58 5.3.16 Datex-Ohmeda Oxygen Saturation module, M-OSAT ... 59 5.3.17 BIS-Module, M-BIS... 59 5.3.18 Entropy Module, M-ENTROPY... 59 5.3.19 Memory Module, M-MEM ... 59 5.3.20 Recorder Module, M-REC ... 59 5.3.21 Network Board, B-NET and UPINET board, B-UPI4NET... 60 5.3.22 Interface Board/Module, B-INT/M-INT ... 60 5.3.23 Device Interfacing Solution, N-DISxxx... 60 5.3.24 General ... 60
General troubleshooting
61
APPENDICES A, B
63
Functional Check Form
1
Appendix B
1
ElectroMagnetic Compatibility
1
iii Document No. 8005796
Datex-Ohmeda S/5 Anesthesia Monitor and S/5 Critical Care Monitor
TABLE OF FIGURES Figure 1
S/5 AM with D-VMC15 monitor and S/5 CCM with D-LCC15... 1
Figure 2
General bus structure of S/5 system ... 11
Figure 3
Distributed processing in S/5 system... 12
Figure 4
Principle of UPI board operation... 12
Figure 5
Software loading... 13
Figure 6
General structure of parameter modules ... 13
Figure 7
Central Unit: S/5 8-Module Frame, F-CU8... 15
Figure 8
Parameter module insertion... 16
Figure 9
Rear view and PC board positioning ... 17
Figure 10
Service reset button ... 18
Figure 11
Display options... 19
Figure 12
Brightness and contrast controls, D-VNC15... 22
Figure 13
Address dip switch settings, B-DISPX ... 26
Figure 14
Resolution dip switch settings, B-DISPX ... 26
Figure 15
Address jumper settings, B-DISP rev. 00 ... 27
Figure 16
Address jumper settings, B-DISP rev. 01 (or higher) and B-DISP19... 27
Figure 17
AUTO/VGA resolution jumper settings, B-DISP rev. 01, or higher and B-DISP19 ... 27
Figure 18
Address jumper settings B-DVGA board, rev. 01 and B-DHIGH board, rev. 01 (s/n < 174671) ... 27
Figure 19
Jumper settings, B-DVGA board, rev. 02-03, and B-DHIGH board, rev. 01-02 (s/n > 174670)... 28
Figure 20
Resolution selection logic, B-DISPX rev.00... 28
Figure 21
Airway Module, G-XXXX... 29
Figure 22
Exhaust to reservoir tube ... 30
Figure 23
Exhaust to scavenging tube ... 30
Figure 24 Barcode Reader connected to Central Unit ... 31 Figure 25 Barcode Reader connected to LCD Display ... 31 Figure 26
N-SCAN Barcode Reader connection directly to the keyboard... 32
Figure 27
S/5 Extension Frame, F-EXT4 ... 32
Figure 28
Connecting the interface connector cables to Interface Board, B-INT... 37
Figure 29
Connection cables and LED indicators ... 41
Figure 30
An example of interfacing external devices with Device Interfacing Solution ... 42
Figure 31
S/5 monitor’s general troubleshooting flowchart ... 61
iv Document No. 8005796
General Service Guide
1
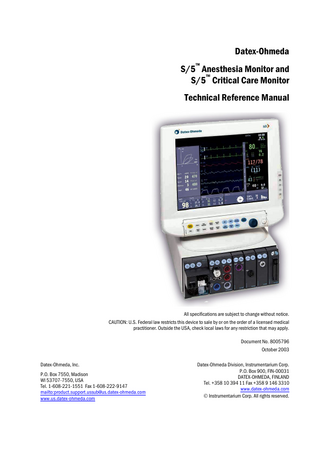
INTRODUCTION The Datex-Ohmeda S/5 Anesthesia Monitor is a modular multiparameter patient monitor used during anesthesia in operating rooms. The Datex-Ohmeda S/5 Critical Care Monitor provides a full patient profile throughout the care period. The modular design provides a flexible system that is easy to upgrade. In addition to parameter changes, the modularity includes an easy upgrade to anesthesia record keeping, monitor networking and interfacing with other external devices.
Figure 1
S/5 AM with D-VMC15 monitor and S/5 CCM with D-LCC15
1 Document No. 8005796
Datex-Ohmeda S/5 Anesthesia Monitor and S/5 Critical Care Monitor
1.1 Notes to the reader This Technical Reference Manual is intended for service personnel and engineers who will perform service and maintenance procedures on the Datex-Ohmeda S/5 Anesthesia Monitor and the Datex-Ohmeda S/5 Critical Care Monitor. This Technical Reference Manual is divided into two parts in one folder: •
Document number 8005796 is the main document of the Anesthesia Monitor and Critical Care Monitor technical reference manual.
•
Part I gives the reader an overview of the S/5 Anesthesia Monitor and S/5 Critical Care Monitor and all monitor specific components e.g. Central Unit and Video Displays. This part of the manual also contains the information needed to installing, interfacing and troubleshooting monitors. Instructions for service procedures, functional check and planned maintenance are also included. Read the manual through and make sure that you understand the procedures described before installation of the monitor. To avoid risks concerning safety or health, strictly observe the warning indications. If you need any assistance concerning the installation, please do not hesitate to contact your authorized distributor.
•
Part II gives detailed descriptions of each component of the S/5 AM, CCM monitor specific parts and different boards. Service check for each product is included in these slots.
For information of other parameter modules, record keeping keyboard, Remote Controllers and Device Interfacing Solution see Technical Reference Manual S/5 Modules, order code 8005674. The manufacturer reserves the right to change product specifications without prior notice. Although the information in this manual is believed to be accurate and reliable, the manufacturer assumes no responsibility for its use. Datex-Ohmeda assumes no responsibility for the use or reliability of its software in equipment that is not furnished by Datex-Ohmeda.
1.1.1 Related documentation S/5 Anesthesia Monitor For more specific information about the clinical aspects and technical background refer to: S/5 Anesthesia Monitor, User’s Guide S/5 Anesthesia Monitor, User’s Reference Manual S/5 Modules Technical Reference Manual For more specific information about other devices closely related to the S/5 Anesthesia Monitor refer to: S/5 Central and Network, User’s Reference Manual Anesthesia Record Keeping Solution, user documentation
S/5 Critical Care Monitor For more specific information about the clinical aspects and technical background refer to: S/5 Critical Care Monitor, User’s Guide S/5 Critical Care Monitor, User’s Reference Manual S/5 Modules Technical Reference Manual S/5 Telemetry System User’s Reference Manual 2 Document No. 8005796
General Service Guide
1.1.2 Conventions used Throughout this manual, the following conventions are used to distinguish procedures or elements of text:
"
Sign the check form after performing the procedure.
Hard Keys
Hard key names on the Command Board, the Remote Controller, and modules are written in bold D-O Sans (12 pt) typeface, e.g. ECG.
Menu Items
Menu items are written in bold italic, D-O Sans (11 pt) typeface, e.g. ECG Setup.
‘Messages’
Messages displayed on the screen are enclosed in single quotes, e.g. ‘Please wait’.
Chapters
When referring to different chapters in the same manual, the chapter name is written in italic typeface and is enclosed in double quotes, e.g. chapter “Cleaning and Care.”
Other documents When referring to different documents, the document name is written in italic typeface, e.g. refer to User’s Reference Manual. Hypertext links
Hypertext links on PDF versions are written in blue color.
WARNING
Warnings are written in bold typeface (13 pt), for example:
WARNING
The 17” display is wall-mountable only. The display must be mounted at 180 cm /71 inch or higher level to prevent any liquid from entering the display casing.
CAUTION
Cautions are written in the following way (13 pt):
CAUTION
The circuit boards contain sensitive integrated circuits that can be damaged by an electrostatic discharge. Careful handling of the boards is therefore essential.
3 Document No. 8005796
Datex-Ohmeda S/5 Anesthesia Monitor and S/5 Critical Care Monitor
1.2 Symbols 1.2.1 Symbols on transport packaging The contents of the transport package are fragile and must be handled with care.
Indicates the correct upright position of the transport package.
The transport package must be kept in a dry environment.
Indicates the temperature limitations within which the transport package should be stored.
1.2.2 Symbols on equipment This battery contains lead acid, and in the event of disposal must be separated from other waste according to local regulations. Pb
This battery contains Pb and can be recycled. Pb
Dangerous voltage. When using the ARK Barcode Reader, N-SCAN, do not stare into beam. The N-SCAN Barcode Reader is a Class 2 laser product.
4 Document No. 8005796
General Service Guide
Attention, consult accompanying documents. When displayed next to an O2 value, indicates that the FiO2 low alarm limit is set below 21 %. When displayed next to an HR value, indicates that the pacer is set on R or a wide QRS is selected. On the 15” display, D-VMC15, indicates that the display should be supplied from the Central Unit, F-CU8, or from the mains outlet. On the 15” display, D-VNC15, indicates that the display must be supplied from the Central Unit, F-CU8, or from the mains outlet via an appropriate additional separating transformer. On the 15” display, D-LCC15, it indicates that the display must be used only together with the original D-LCC15 power adapter. The display should be supplied from the Central Unit, F-CU8, or from the mains outlet. On the 17” display, D-LCC17 it indicates that the display must be used only together with the original D-LCC17 power adapter. On the 17” display, D-VHC17 rev.00-01, indicates that the display must only be supplied from the mains outlet, not from the Central Unit, F-CU8. On the 17” display, D-VHC17 rev. 02 or higher, indicates that the display should be supplied from the Central Unit, F-CU8, or from the mains outlet via an appropriate additional separating transformer. On the 19” display, D-LCC19, indicates that the display must only be supplied from the mains outlet via an appropriate additional separating transformer and the original D-LCC19 power adapter, not from the Central Unit, F-CU8. On the 21” display, D-VSC21, indicates that the display must only be supplied from the mains outlet via an appropriate additional separating transformer, not from the Central Unit, F-CU8. On the Interface Module, M-INT, indicates that the connection is for external devices and not for patient cables. On the Tonometry Module, M-TONO, indicates that use only Tonometrics catheters. On the BIS Module M-BIS, indicates that the converter must not be opened for any reason or autoclaved. On the rear panel of the Central Unit, F-CU8, indicates the following warnings and cautions: − Electric shock hazard. Do not open the cover or the back. Refer servicing to
qualified personnel. − For continued protection against fire hazard, replace the fuse only with one of the
same type and rating. − Disconnect the power supply before servicing.
Type BF (IEC-60601-1) protection against electric shock. Type BF (IEC-60601-1) defibrillator-proof protection against electric shock. Type CF (IEC-60601-1) protection against electric shock. 5 Document No. 8005796
Datex-Ohmeda S/5 Anesthesia Monitor and S/5 Critical Care Monitor
Type CF (IEC-60601-1) defibrillator-proof protection against electric shock. When displayed on the upper left-hand corner of the screen, indicates that the alarms are silenced. When displayed on the menu or in digit fields, indicates that the alarm source has been turned off. Other symbols Equipotentiality. Monitor can be connected to potential equalization conductor. Alternating current. Fuse. Connector for color display. Display power supply output. 15" LCD display video signal input (version 02) 15" LCD display power input (version 02) 15" LCD display video signal input (version 03) 15" LCD display power input (version 03) SN, S/N
Serial Number. Sub menu. Selecting this symbol on a menu opens a new menu. The monitor is connected to the Datex-Ohmeda Network. The data card (green) and/or the Menu card (white) is inserted. Indicates beats are detected. Respiration rate is measured using impedance respiration measurement. In this manual indicates the procedure for making selections from the menus. ESD warning symbol for electrostatic sensitive devices. Pins of connectors identified with the ESD warning symbol should not be touched. Connections should not be made to these connectors unless ESD precautionary procedures are used. See "Safety precautions: ESD precautionary procedures" in the "User's Reference Manual" for details. Symbol for non-ionizing electromagnetic radiation. Interference may occur in the vicinity of equipment marked with the symbol.
6 Document No. 8005796
General Service Guide
1.3 Safety 1.3.1 Classification Classification according to IEC 60601-1 • • • •
CLASS 1 equipment according to the type of protection against electrical shock. TYPE BF or CF equipment according to the degree of protection against electrical shock is given in the specification for each parameter module. Equipment not suitable for use in the presence of flammable anesthetic mixture with air or with oxygen/nitrous oxide. Continuous operation according to the mode of operation.
Classification according to IEC 60529 Degree of protection against the harmful ingress of water as detailed in IEC 60529: IPX0.
1.3.2 Responsibility of the manufacturer Datex-Ohmeda Division, Instrumentarium Corp. is responsible for the safety, reliability and the performance of the software and equipment only if: • Assembly, operations, extensions, readjustments, modifications, service and repairs are carried out by personnel authorized by Datex-Ohmeda. • The electrical installation of the monitor room complies with appropriate requirements. • The system is serviced, maintained and used in accordance with the Technical Reference Manual.
1.3.3 Safety precautions Warnings WARNING
A WARNING indicates a situation in which the user or the patient may be in danger of injury or death.
Power connection •
Before connecting the power cord to the mains outlet, check that the local voltage and frequency correspond with the rating stated on the device plate on the rear panel of Central Unit, F-CU8, and the Video Display, D-VHC17. See instructions for different displays from section “Displays”.
•
Connect the monitor to a three-wire, grounded, hospital grade socket. Do not remove the grounding pin from the power plug.
•
Use only an intact power cord. Replace the power cord if it is cracked, frayed, broken or otherwise damaged.
•
Do not apply tension to the power cord otherwise the cord may get damaged.
•
Do not use extension cords or adapters of any type.
7 Document No. 8005796
Datex-Ohmeda S/5 Anesthesia Monitor and S/5 Critical Care Monitor Laser radiation •
When using the ARK Barcode Reader, N-SCAN, do not stare into beam. The N-SCAN is a Class 2 laser product.
External connection •
Do not connect any external devices to the monitor other than those specified by DatexOhmeda.
Fuse replacement •
Replace a fuse only with one of the same type and rating.
•
Do not use the monitor in the presence of flammable anesthetics.
•
Do not perform any testing or maintenance on the monitor while it is being used on a patient.
•
Use only cables and accessories approved by Datex-Ohmeda. Do not modify them. Other cables and accessories may damage the monitor or interfere with the measurement.
•
PACEMAKER PATIENTS: The impedance respiration measurement may cause rate changes in Minute Ventilation Rate Responsive Pacemakers. In this case set the pacemaker rate responsive mode off or turn the monitor impedance respiration measurement off.
Explosion hazard
Patient safety
Cleaning and service •
Only trained personnel with proper tools and test equipment should perform the tests and repairs described in this manual. Unauthorized service may void the monitor warranty.
•
Turn the power off and unplug the power cord before cleaning or service. Completely remove any moisture before reconnecting the power cord to the mains outlet.
•
Do not touch any exposed wire or conductive surface while any cover is removed and the monitor is energized. The voltages present can cause injury or death.
•
Always perform an electrical safety check and a leakage current test on the monitor after service.
•
WARNING: Use only cables, transducers and accessories approved by Datex-Ohmeda. Other cables, transducers and accessories may damage the system, result in increased emissions or decreased immunity of the system or interfere with the measurement. Singleuse accessories are not designed to be reused. Reuse may cause a risk of contamination and affect the measurement accuracy.
Accessories
Cautions CAUTION
A CAUTION indicates a condition that may lead to equipment damage or malfunction.
Installation 8 Document No. 8005796
General Service Guide •
Leave a space behind the monitor to allow proper ventilation.
•
Ensure that the module is properly orientated (i.e. module release latch facing downward) before insertion.
•
Allow two minutes for warm-up and note any error messages or deviations from normal operation.
•
Clean the rear panel fan dust filter once a month or whenever necessary.
•
Do not connect a sampling line to the female Patient Spirometry connector while the other end of the sampling line is connected to the D-fend water trap. The pressure in the gas sampling system may cause damage to the PVX unit pressure transducers.
Before use
Autoclaving and sterilizing •
Do not autoclave any part of the monitor.
•
Do not gas sterilize the modules.
Cleaning and service •
Do not use ammonia, phenol, or acetone based cleaners. These cleaners may damage the monitor surface.
•
Do not immerse the monitor in any liquid. Do not allow liquid to enter the monitor or modules.
•
Electrostatic discharge through the PC boards may damage the components. Before handling PC boards, wear a static control wrist strap. Handle all PC boards by their nonconductive edges and use anti-static containers when transporting them.
•
Do not break or bypass the patient isolation barrier when testing PC boards.
•
Do not clean the spirometry tubes with high pressure air or O2 flushing while the spirometry tubes are connected to Patient Spirometry connector. High differential pressure may damage PVX unit pressure transducers.
Special components •
Special components that are used in these monitors are vital to assure reliability and safety. Datex-Ohmeda assumes no responsibility for damage if replacement components not approved by Datex-Ohmeda are used.
•
A lithium battery on the CPU Board. Dispose of the faulty IC containing the battery according to local regulations.
Batteries The battery package in the power supply unit contains lead acid (Pb) which is hazardous to the environment and therefore needs to be disposed of carefully according to local regulations. To replace the batteries safely, please refer to the instructions in this manual. •
Do not short-circuit the battery terminals, this may produce a very high current, which will damage the battery.
9 Document No. 8005796
Datex-Ohmeda S/5 Anesthesia Monitor and S/5 Critical Care Monitor •
Do not dispose of the battery into open flame, nor put the battery near fire, as it may explode.
•
Do not dismantle the battery. It contains electrolyte, which may damage clothing or cause injury to skin or eyes. If exposed to electrolyte, wash the injured area with plenty of clean water and contact a doctor.
See also section “Symbols”. Storage and transport Do not store or transport the monitor outside the specified temperature and pressure ranges: Temperature Ambient pressure Humidity except D-VSC21
-10...+50 °C/14...122 °F 660...1060 hPa/500...800 mmHg/660...1060 mbar 10...90 % non-condensing 20...80 % non-condensing
Discard Dispose of any device or parts according to local regulations. The manufacturer accepts no responsibility for any modifications made to the monitor outside its factory.
10 Document No. 8005796
General Service Guide
2
SYSTEM DESCRIPTION
2.1 Introduction Datex-Ohmeda S/5 monitors build up a freely configurable modular system. The architecture is designed to enable different module combinations so that the user is able to get the desirable parameter and feature set. This modular approach makes it possible to add new features when they are needed.
2.2 Bus structure The operation of Datex-Ohmeda S/5 monitors is based on two communication channels, the CPU bus and the module bus. All boards connected to the CPU bus, as well as the parameter modules attached to the module bus, receive power from the same power supply, which is an integral part of the Central Unit, F-CU8.
UPI
Display Controller
CPU
Power Supply Unit
CPU Bus
Module Bus
Parameter Module
Figure 2
Parameter Module
Parameter Module
The CPU bus is a parallel communication channel used only for internal data transfer between the boards connected to one Central Unit. It is based on the ISA bus used in IBM PC computers. Data is transferred on this 16 bit wide bus using the CPU clock frequency. The module bus is used to connect the parameter modules to the Central Unit. It based on the widely used industry standard RS-485, which uses a differential serial method to transfer data. This type of bus is robust and it allows parameter modules to be inserted or removed while the power is on. The module bus uses a 500 kbps data transfer rate and can be used for longer distances than the CPU bus, e.g. for external frame connections.
General bus structure of S/5 system
The RS-485 type serial communication supports so-called multidrop or party line connections. This means that all parameter modules connected to module bus use the same two wires for communication purposes. The advantage of this is that all module bus connectors are identical and the parameter modules can be connected in any order and position.
2.3 Distributed processing A system assembled from S/5 products is a multiprocessor system. All parameter modules have their own microprocessor, which performs low-level functions such as module key control, waveform filtering and pneumatic control, etc. At the same time the main CPU performs higher level tasks such as trending and alarm control. While the parameter modules and CPU are performing their tasks, the UPI (Universal Peripheral Interface) microprocessor handles all functions needed to transfer data between the parameter modules and the CPU. At the same time the microprocessor on the Display Controller board performs pixel calculations for graphics. 11 Document No. 8005796