Dräger Medical
Infinity Monitoring System
Infinity M540 Patient Monitor SW VG7.1 Ed1 Instructions for Use May 2020
Instructions for Use
440 Pages
Preview
Page 1
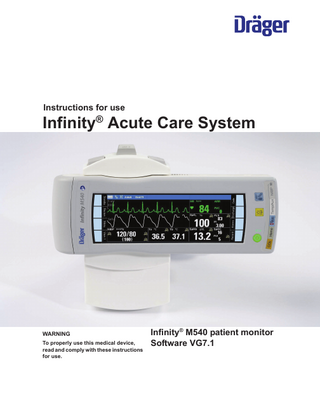
Instructions for use
Infinity® Acute Care System
WARNING To properly use this medical device, read and comply with these instructions for use.
Infinity® M540 patient monitor Software VG7.1
Typographical conventions 1
Consecutive numbers indicate steps of action, with the numbering restarting with 1 for each new sequence of actions.
Bullet points indicate individual actions or different options for action. –
Dashes indicate the listing of data, options, or objects.
(A) Letters in parentheses refer to elements in the related illustration. A
Letters in illustrations denote elements referred to in the text.
>
The greater-than symbol indicates the navigation path in a dialog. Bold, italicized text indicates labels on the device and texts that are displayed on the screen.
Figures Images of products and screen content in this document may differ from the actual products depending on configuration and design.
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
3
Trademarks –
Infinity®
–
Innovian®
is a trademark of BODE Chemie GmbH.
–
®
MCable
–
Buraton®
–
Medical Cockpit®
–
Mikrozid®
–
MPod®
–
perform®
–
MonoLead®
–
acryl-des®
–
Tcore®
are trademarks of Schülke & Mayr. GmbH.
–
Dismozon®pur
are trademarks of Dräger.
–
–
Masimo®
is a trademark of Ecolab.
–
SET
–
–
PVI®
–
SpCO
–
SpHb®
is a trademark of Antiseptica.
–
SpMet
–
–
Pulse CO-Oximeter Signal Extraction®
®
OxyCide®
BruTab 6S®
is a trademark of Brulin. –
®
®
Descogen®
Klorsept®
is a trademark of Medentech.
are trademarks of Masimo corporation.
–
Virkon®
–
CapnoLine®
is a trademark of DuPont.
–
Capnostream®
–
Microcap®
–
MicroPod®
–
Microstream®
–
Oridion®
–
FilterLine®
are trademarks of Oridion Medical 1987 Ltd. –
Medtronic®
–
Nellcor®
–
OxiMax®
–
SatSeconds®
are trademarks of a Medtronic company.
4
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
Microstream® MicroPod® External Capnography Module patents The capnography component of this product is covered by one or more of the following US patents: 6,437,316; 6,428,483; 6,997,880; 7,488,229; 8,414,488; 8,412,655 and their foreign equivalents. Additional patent applications pending.
Open-source software Dräger devices that use software may use open-source software, depending on their setup. Open-source software may be subject to different terms of license. Additional information regarding
the open-source software used in this device is available at the following web page: www.draeger.com/opensource
Safety information definitions WARNING A WARNING statement provides important information about a potentially hazardous situation which, if not avoided, could result in death or serious injury. CAUTION A CAUTION statement provides important information about a potentially hazardous situation which, if not avoided, may result in minor or moderate injury to the user or patient or in damage to the medical device or other property. NOTE A NOTE provides additional information intended to avoid inconvenience during operation.
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
5
Target Groups
Duties of the operating organization
Reprocessing personnel
The tasks described in this document specify the requirements that have to be met by each respective target group.
Task
Requirement
Reprocessing
Specialist knowledge in the reprocessing of medical devices
The operating organization of this product must ensure the following: The target group has the required qualifications (e.g., has undergone specialist training or acquired specialist knowledge through experience).
Service personnel Task
Requirement
The target group has been trained to perform the task.
Installation
Specialist knowledge in electrical engineering and mechanics
Description of target groups
Basic service activities (inspection, maintenance according to "Maintenance" on page 377).
The target groups may only perform the following tasks if they meet the corresponding requirements.
Specialized service personnel
The target group has read and understood the chapters required to perform the task.
User Task
Requirement
Use of the product in Specialist medical accordance with the knowledge in the use of the intended use product
Experience in the servicing of medical devices
Task
Requirement
Installation
Specialist knowledge in electrical engineering and mechanics
Basic and complex service activities (inspection, Experience in the servicing maintenance, repair) of medical devices Training in service activities on this product A service contract with Dräger is recommended.
Abbreviations and symbols For explanations, refer to the sections "Abbreviations" on page 36 and "Symbols" on page 33. 6
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
Contents
Contents Contents...
7
For your safety and that of your patients. . . 11 General safety information... 16 Application... 21 Intended use... 22 Indications for use... 23 Overview... 25 Overview... 26 M500 docking station... 29 Additional hardware... 30 Symbols... 33 Abbreviations... 36 Assembly and preparation... 39 Overview... 40 Docking/undocking the M540... 41 Locking/unlocking the M540... 42 Connecting the system cables in an IACS configuration... 43 Connecting the system cable in an M540 stand-alone configuration... 43 Additional M540 accessories... 43 Mounting the Infinity MCable – Masimo SET and Masimo rainbow SET/Nellcor OxiMax... 44 Operating concept... 47 Overview... 48 M540 in standalone / wireless mode... 49 M540 in an IACS configuration... 52 Communicating with the Infinity network... 52 ICS (Infinity CentralStation) communication . . . 53 Remote view and remote control... 56 Function keys... 59 Monitoring area... 60 Adjusting the display... 64 Battery power... 65 Power-saving mode... 66 Views... 66 Profiles... 67 Saving a profile... 80
Profile behavior in an IACS configuration... 80 Profile behavior in a standalone configuration 81 Standby mode... 83 Privacy mode... 83 Recordings/reports... 84 Getting started...
87
Overview of monitoring a patient... 88 Turning the M540 on/off... 88 Admitting a patient... 89 Discharging a patient... 90 Patient categories... 91 Alarms...
93
Overview of alarms... 94 Alarm priorities... 94 Alarm processing... 95 Activating or deactivating alarm validation . . . 96 Optical alarm signals... 97 Acoustic alarm signals... 98 Testing visual and acoustic alarm signals... 100 Special alarm behavior... 100 Pre-silencing alarms... 105 Pausing acoustic alarm signals (audio pause) 106 Pausing alarm monitoring temporarily... 108 Activating or deactivating alarm monitoring. . . 109 Configuring a patient’s alarm settings... 110 Event recall... 113 Viewing a snapshot of a single event... 115 Configuring the SpO2 alarm priority... 116 Alarm management setup (password-protected) 117 The Code function key... 117 Alarm groups... 117 Alarm ranges and defaults... 118 ECG, arrhythmia, and ST segment... 131 Overview of ECG and heart rate monitoring . . 133 ECG precautions... 134 Connecting the 3-, 5-, 6-wire lead sets for ECG monitoring... 135 Connecting the lead sets for 12-lead ECG monitoring... 136
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
7
Contents
Connecting the lead sets for neonatal ECG monitoring... 137 Patient preparation for ECG monitoring... 138 ECG display... 139 ECG electrode colors... 140 Electrode placement for adult and pediatric patients 141 Electrode placement for neonates... 143 12-lead monitoring... 144 Accessing the ECG dialog... 144 ECG parameter setup functions... 145 Monitoring paced patients... 150 Pacemaker precautions... 151 Optimizing pacer processing... 154 Arrhythmia monitoring overview... 154 Selecting arrhythmia leads... 155 Arrhythmia processing... 156 Arrhythmia modes... 157 Arrhythmia display... 159 Accessing the arrhythmia dialog... 160 Arrhythmia parameter setup functions... 160 Monitoring ST overview... 161 Standard ST monitoring... 161 TruST 12-lead monitoring... 162 12-lead ST monitoring... 162 Connecting lead sets for ST monitoring... 162 ST display... 163 ST complex dialogs... 164 ST measuring points... 165 ST reference... 165 Accessing the ST dialog... 166 ST setup functions... 166 Learning/relearning QRS pattern... 167
SpO2 and Pulse CO-Ox monitoring with Masimo SET MCable... 181
Impedance respiration (RRi)... 169
Overview... 214 Non-invasive blood pressure precautions... 215 Patient preparation for non-invasive blood pressure monitoring... 217 Non-invasive blood pressure display... 219 Non-invasive blood pressure measurement modes 220 Venous stasis... 222 Accessing the non-invasive blood pressure dialog 223 Non-invasive blood pressure parameter setup functions... 224
Overview of respiration monitoring... 170 RRi precautions... 170 Connecting the 3-, 5-, 6-wire lead sets for respiration monitoring... 171 Connecting the lead sets for 12-lead respiration monitoring... 172 Connecting the lead wires for neonatal respiration monitoring... 173 Patient preparation for respiration monitoring . 174 Respiration display... 176 Respiration measuring modes... 177 Accessing the respiration dialog... 177 Respiration parameter setup functions... 178 8
Overview of SpO2 monitoring... 182 SpO2 and Pulse CO-Ox precautions... 184 Connecting the Masimo SET MCable... 186 Connecting the Masimo rainbow SET MCable 187 Patient preparation... 188 SpO2 and Pulse CO-Ox display... 189 Accessing the SpO2 dialog... 191 SpO2 parameter setup functions... 192 Pulse CO-Ox parameter setup functions... 194 Password-protected Masimo rainbow SET setup functions... 196 SpO2 and pulse rate monitoring with Nellcor OxiMax MCable... 197 Overview of SpO2 monitoring... 198 SpO2 precautions... 198 Connecting the Nellcor OxiMax MCable... 200 Patient preparation for SpO2 monitoring... 201 SpO2 display... 202 Accessing the SpO2 dialog... 202 SpO2 parameter setup functions... 203 Temperature... 205 Overview of temperature monitoring... 206 Precautions... 206 Connecting the temperature sensors... 207 Temperature display... 209 Accessing the temperature dialog... 210 Temperature parameter setup functions... 211 Non-invasive blood pressure (NIBP)... 213
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
Contents
Invasive pressures (IP)... 225 Overview of invasive pressure monitoring... 226 Invasive pressure precautions... 228 Connecting the Hemo4 and Hemo2 pods... 229 Connecting the MPod – Quad Hemo... 230 Connecting a second MPod – Quad Hemo . . . 231 Connecting the Dual Hemo MCable... 233 Patient preparation for invasive pressure monitoring... 234 Invasive pressure display... 234 Labeling Invasive pressure channels... 236 Standard pressure labels... 236 Pressure label conflicts... 238 Zeroing a pressure transducer... 238 Pulmonary wedge pressure... 241 Accessing the invasive pressure dialog... 241 Invasive pressure parameter setup functions. . 242
Calibration check... 276 Scio Monitoring... 277 Overview of Scio monitoring... 278 Connecting and disconnecting the Scio module 280 Accessing Scio settings... 282 CO2 settings... 283 O2 settings... 284 N2O settings... 285 Agent settings... 286 CO2 display... 288 O2 display... 291 N2O display... 292 Agent display... 293 xMAC (MAC multiple)... 295 Zeroing the gas analyzer... 296
Cardiac output... 245
System configuration... 297
Overview of cardiac output monitoring... 246 Cardiac output precautions... 246 Connecting the cardiac output hardware... 247 Patient preparation for cardiac output monitoring 249
Overview... 298 Configuring general settings... 299 Configuring the patient settings... 301 Configuring the system settings... 302 Viewing the system information... 308 Configuring the biomed settings... 310 Configuring the screen layout... 316 Configuring alarm settings... 317 Configuring the battery alarm... 320 Options... 320
Mainstream CO2 monitoring... 251 Overview of mainstream CO2 monitoring... 252 CO2 precautions... 252 Connecting the CO2 sensor... 254 Patient preparation for CO2 monitoring... 255 CO2 display... 255 Using the CO2 dialog... 258 CO2 parameter setup... 259 Performing a calibration check... 261 Microstream CO2 monitoring... 263 Overview of Microstream CO2 monitoring... 264 Precautions... 266 Connecting the Microstream MCable... 268 Additional Microstream MCable mounting options 269 Detaching Microstream MCable and components 271 Choosing a sample line... 272 CO2 display... 272 Using the CO2 dialog box... 274 CO2 parameter setup... 275
Troubleshooting... 323 Overview... 324 Device communication messages / general device messages... 324 M540 battery messages... 326 Messages... 326 ECG... 328 ST... 331 Arrhythmia... 332 Respiration (RRi)... 333 SpO2... 335 Non-invasive blood pressure... 340 Cardiac output... 342 Temperature... 343 Invasive pressure... 344 Mainstream CO2... 346 Scio... 353
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
9
Contents
Reprocessing... 365 Reprocessing overview... 366 Reprocessing of M540 device-specific components 366 Reprocessing of accessories... 371 Disposal... 375 EU Directive 2002/96/EC (WEEE)... 376 Maintenance... 377 Overview... 378 Inspection... 379 Visual inspection... 379 Inspection / safety checks... 380 Preventive maintenance... 381 Technical data... 383 Overview... 384 System compatibility... 384 Device combinations... 385 Infinity M540... 386 Infinity M500... 390 Power supply (PS50)... 391 120 Watt desktop power supply (PS120)... 392 Infinity MCable – Masimo SET and Infinity MCable – Masimo rainbow SET... 395 Infinity MCable – Nellcor OxiMax... 396 Infinity Hemo2 and Hemo4 pods... 397 Infinity MPod – Quad Hemo... 398 Infinity MCable – Dual Hemo... 399 Infinity MCable – Analog/Sync... 400 Infinity MCable – Nurse call... 402 Parameter monitoring specifications... 403 Electromagnetic compatibility... 422 EMC declaration... 427 Index... 429
10
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
For your safety and that of your patients
For your safety and that of your patients Mandatory reporting of adverse events... 12 Strictly follow these instructions for use... 12 Storing the instructions for use... 12 Training... 12 Maintenance... 12 Safety checks... 13 Accessories... 13 Installing accessories... 13 Sterile accessories... 13 Restrictions for use... 13 Restriction of distribution... 13 Connected devices... 14 Safe connection with other electrical equipment... 14 Connection to hospital network... 14 Patient safety... 14 Patient monitoring... 15 General safety information... 16 Not for use in areas of explosion hazard... 17 Information on electromagnetic compatibility . . 17 Operating location... 17 Defibrillator precautions... 18 Electrosurgery... 18 Security recommendations... 19
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
11
For your safety and that of your patients
Mandatory reporting of adverse events
Storing the instructions for use
Serious adverse events with this product must be reported to Dräger and the responsible authorities.
WARNING Risk of incorrect use
Strictly follow these instructions for use NOTE The Infinity Acute Care System provides the following additional instructions for use: –
Infinity Acute Care System – Monitoring applications (describes the Cockpit user interface of the IACS)
–
Infinity Acute Care System – Medical Cockpit (describes the hardware of the Cockpit)
–
Infinity Acute Care System – Monitoring accessories (describes all of the IACS accessories).
Please refer to these additional instructions for use for device-specific information. WARNING Risk of incorrect operation and of incorrect use. Any use of the medical device requires full understanding and strict observation of all sections of these instructions for use. The medical device must only be used for the purpose specified under "Application," and in conjunction with appropriate patient monitoring. Strictly observe all WARNING and CAUTION statements throughout these instructions for use and all statements on medical device labels. Failure to observe these safety information statements constitutes a use of the medical device that is inconsistent with its intended use.
12
Instructions for use must be kept accessible for the user.
Training Training for users is available from the responsible Dräger organization, see www.draeger.com.
Maintenance WARNING Risk of medical device failure and of patient injury. The medical device must be inspected and serviced regularly by service personnel. Repair and complex maintenance carried out on the medical device must be performed by experts. If the above is not complied with, medical device failure and patient injury may occur. Observe chapter "Maintenance." A service contract with Dräger is recommended. It is recommended that original Dräger parts are used for repairs and that Dräger performs these repairs. WARNING Any modification of this device or any use different from the one specified in these instructions for use may cause interference with other equipment or result in injury to the patient or the user, including electric shock, burns or death.
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
For your safety and that of your patients
Safety checks
Installing accessories
The medical device must be subject to regular safety checks. See chapter “Maintenance“.
CAUTION Risk of device failure
Accessories
Install accessories to the basic device in accordance with the instructions for use of the basic device. Make sure that there is a safe connection to the basic device.
WARNING Risk due to incompatible accessories. Dräger has only tested the compatibility of accessories listed in the current list of accessories. If other accessories are used, there is a risk of patient injury due to medical device failure. Dräger recommends that the medical device is only used with accessories listed in the current list of accessories.
CAUTION The MPods and associated accessories that have patient contact are not manufactured with natural rubber latex.
Strictly observe instructions for use and assembly instructions.
Sterile accessories CAUTION Risk of medical device failure and of patient injury. Do not use sterile-packaged accessories if the packaging has been opened, is damaged, or if there are other signs of non-sterility. Single-use accessories must not be reused, reprocessed, or resterilized.
Restrictions for use
WARNING To avoid electric shock, the equipment should only be connected to a power source that is properly grounded (protective earth ground). CAUTION Device for use in healthcare facilities only and exclusively by persons as defined in the target groups (see page 6).
Restriction of distribution Federal Law (U.S.) restricts this device to sale by or on the order of a physician.
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
13
For your safety and that of your patients
Connected devices
Connection to hospital network
WARNING Risk of electric shock and of device malfunction
Many medical devices manufactured by Dräger use networks to transmit patient data in real-time and to notify clinical users of alarm conditions. Hospitals should refer to IEC 80001-1 before attempting to connect such medical devices to their IT networks.
Any connected devices or device combinations not complying with the requirements mentioned in these instructions for use can compromise the functional integrity of the medical device and lead to electric shock. Before operating the medical device, strictly comply with the instructions for use of all connected devices and device combinations.
Safe connection with other electrical equipment
Patient safety The design of the medical device, the accompanying documentation, and the labeling on the medical device are based on the assumption that the purchase and the use of the medical device are restricted to persons familiar with the most important inherent characteristics of the medical device. Instructions and WARNING and CAUTION statements are therefore largely limited to the specifics of the Dräger medical device.
WARNING Risk of patient injury
These instructions for use do not contain any information on the following points:
Electrical connections to equipment not listed in these instructions for use should only be made following consultation with the respective manufacturers. Equipment malfunction may result and pose a risk of patient injury.
–
Risks that are obvious to users
–
Consequences of obvious improper use of the medical device
–
Potentially negative effects on patients with different underlying diseases
WARNING The leakage current increases when multiple medical devices are connected to a patient. Make sure that the galvanic isolation of each device is suitable for the intended application. Connect only equipment to the analog and digital signal inputs and outputs that is setup and tested according to IEC standards.
Medical device modification or misuse can be dangerous. CAUTION Risk of patient injury Do not make therapeutic decisions based solely on individual measured values and monitoring parameters.
To protect the patient from possible injury due to electrical shock, peripheral devices should only be connected to a monitor within the same room. The installer or service provider should verify that the leakage current of the interconnected system meets the electrical safety requirements of IEC 60601-1.
14
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
For your safety and that of your patients
Patient monitoring The user of the medical device is responsible for choosing a suitable patient monitoring system that provides appropriate information on medical device performance and patient condition. Patient safety may be achieved by a wide variety of means ranging from electronic surveillance of medical device performance and patient condition to direct observation of clinical signs. The responsibility for selecting the best level of patient monitoring lies solely with the user of the medical device.
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
15
For your safety and that of your patients
General safety information The following WARNING and CAUTION statements apply to general operation of the medical device.WARNING and CAUTION statements specific to subsystems or particular features of the medical device appear in the respective sections of these instructions for use or in the instructions for use of another product being used with this medical device.
WARNING To avoid patient injury as the result of a falling monitor when using a rolling trolley, universal bed hook, or handle hook mount, do not apply excessive force to the monitor or mount when entering or exiting elevators, or passing over thresholds or uneven surfaces.
WARNING Risk of explosion and of chemical burns.
CAUTION To avoid injuring the patient, disconnect all sensors that will not be used during transport, before moving the patient.
Improper handling of batteries can result in explosions and chemical burns. Do not throw batteries into fire. Do not force batteries open. WARNING Never use equipment that has been damaged or compromised for patient monitoring. WARNING To avoid electric shock, inspect all cables before use. Never use cables that appear cracked, worn, or damaged in any way (doing so may compromise performance or put the patient at risk). WARNING To avoid risk of electric shock, this equipment must only be connected to a main power source with a protective earth ground. WARNING Do not cover the device with blankets or bed sheets. To prevent burns to the patient, avoid direct contact between external surfaces and the patient.
16
CAUTION Read all cleaning instructions (for example, originating from the disinfectant manufacturer and the hospital) carefully before cleaning the device. Refer to the chapter entitled "Reprocessing" on page 365 for device-specific cleaning instructions. Moisture may damage the circuits, compromise critical performance and present a safety risk. WARNING Dräger recommends using the Infinity Acute Care System or the M540 for primary diagnosis and the ICS (Infinity CentralStation) for patient viewing only. WARNING To prevent restriction of air flow to the device and also prevent potential overheating, do not allow the patient to come into direct contact with system components for extended periods of time.
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
For your safety and that of your patients
For countries subject to the EU directive 2002/96/EC This device is subject to EU Directive 2002/96/EC (WEEE). In order to comply with its registration according to this directive, this device may not be disposed of at municipal collection points for waste electrical and electronic equipment. Dräger has authorized a company to collect and dispose of this device.
Portable and mobile radio frequency communications equipment can affect medical electrical equipment. WARNING
To initiate collection or for further information, visit Dräger on the Internet at www.draeger.com. Use the Search function with the keyword “WEEE” to find the relevant information. If access to Dräger's website is not possible, contact the local Dräger organization.
Do not connect connectors with an ESD warning symbol and do not touch their pins without implementing ESD protective measures. Such protective measures may include antistatic clothing and shoes, touching a potential equalization pin before and during connection of the pins, or using electrically insulating and antistatic gloves. All users concerned must be instructed in these ESD protective measures.
Not for use in areas of explosion hazard
Operating location
WARNING Risk of explosion
Only use devices (monitor, MPod, MCable, and accessories) in areas that meet the environmental requirements outlined in the technical data section.
This medical device is neither approved nor certified for use in areas where oxygen concentrations greater than 25% (combustible or explosive gas mixtures) are likely to occur.
WARNING When placing the device, make sure that adequate airflow exists. To prevent overheating, position the device with at least 5 cm (2 in) of space all around.
Information on electromagnetic compatibility Medical electrical equipment is subject to special precautionary measures concerning electromagnetic compatibility (EMC) and must be installed and put into operation in accordance with the EMC information provided in the Electromagnetic compatibility section in the Technical data chapter.
WARNING To avoid interfering with device operation, do not operate devices (monitor, MPod, MCable, and accessories) close to equipment that emits microwave or other high-frequency emissions. WARNING Periodically make sure that the device is properly mounted and secured to prevent injury. Make sure the requirements for maximum load and slope of floor are met. Consult the documentation of the mounting manufacturer for detailed information. WARNING To minimize the risk of patient strangulation, carefully position and secure sensor cables. Also position the sensor cables to minimize inductive loops.
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
17
For your safety and that of your patients
CAUTION To prevent overheating, do not place the device in direct sunlight or near heaters. CAUTION After extended exposure in a cold environment, acclimate the device carefully so that condensation does not form on the electronic parts and damage the device. CAUTION To avoid damaging the touch-sensitive screen, do not allow sharp instruments to touch the front of the devices. CAUTION To avoid short-circuiting and otherwise damaging the device, Dräger recommends that no fluids come in contact with the IACS devices when they are connected to a power socket. If fluids are accidentally spilled on the equipment, remove the affected device from service as soon as possible and have service personnel verify that patient safety is not compromised.
Defibrillator precautions The M540 and the peripheral devices are protected against high-frequency interference from defibrillators and electrosurgical units and against 50- and 60-Hz power line interference. WARNING To protect the patient during defibrillation and to ensure accurate ECG information, use only ECG electrodes and cables specified by Dräger. Removal of applied parts that are not rated defibrillation-proof, such as disposable SpO2 sensors, may be required to prevent sensor breakdown and energy shunting.
18
CAUTION To prevent burns and electric shock due to the rerouting of electrical current through electrodes, do not position the defibrillator pads near any electrodes or sensors. CAUTION Only defibrillate across the chest.
Electrosurgery Observe the following precautions during electrosurgery to reduce electrosurgical unit (ESU) interference and improve operator and patient safety. WARNING For better performance and to reduce the hazard of burns during surgery, always use accessories designed for ESU environments. Do not use skin temperature sensors. WARNING To reduce the hazard of burns during electrosurgery, keep the sensor or transducer (e.g., ECG, SpO2 pressure) and their associated cables away from the surgical site, the ESU return electrode, and earth ground. NOTE Cover internally placed reusable temperature sensors with temperature sensor sheaths. WARNING To reduce the hazard of burns during electrosurgery, do not use four-lead extension cables in electrosurgical environments.
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
For your safety and that of your patients
Security recommendations Dräger makes the following security recommendations: –
Physical security of the patient monitors is recommended and is the responsibility of the operating organization.
–
Physical security of the telecommunications closet is recommended and is the responsibility of the operating organization.
–
Dräger recommends that operating organizations restrict physical access to unused ethernet ports on the IACS.
–
Dräger recommends that operating organizations restrict physical access to unused USB and serial ports on the IACS.
–
Dräger relies on the medical device isolation mechanism of the VLANs and the proper configuration, implementation, and use of the operating organization's security measures to prevent the introduction of malware onto the Infinity network.
CAUTION Dräger recommends that all documents opened for viewing within a hospital LAN must come from a secure source.
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
19
This page has been left blank intentionally.
20
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
Application
Application Intended use... 22 Indications for use... 23
Instructions for use – Infinity® Acute Care System – Infinity® M540 – VG7.1
21