Fresenius Medical
T688 SERIES Instruction Guide Ver 4 March 2013
Instruction Guide
13 Pages
Preview
Page 1
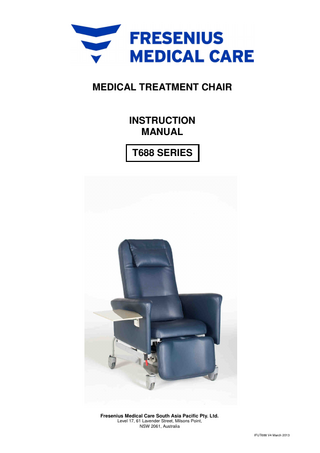
MEDICAL TREATMENT CHAIR
INSTRUCTION MANUAL T688 SERIES
Fresenius Medical Care South Asia Pacific Pty. Ltd. Level 17, 61 Lavender Street, Milsons Point, NSW 2061, Australia IFUT688 V4 March 2013
FRESENIUS MEDICAL CARE
MEDICAL TREATMENT CHAIR
INSTRUCTION MANUAL - T688 Series
CONTENTS 1
INTRODUCTION, PURPOSE & GENERAL SAFETY ISSUES ... 3
2
OPERATING THE MEDICAL TREATMENT CHAIR ... 4
3
INSTALLING THE MEDICAL TREATMENT CHAIR ... 5
4
3.1
WARNINGS AND PRECAUTIONS ... 5
3.2
BATTERY BACKUP (if fitted) ... 6
3.3
HAND CONTROLS & PROGRAMMING ... 6
3.3.1
Hand Control ... 6
3.3.2
Programming Instructions ... 7
3.3.3
Recalibration ... 7
MAINTENANCE ... 8 4.1
INSPECTION & SERVICING ... 8
4.2
CLEANING ... 8
4.3
FABRIC STANDARDS ... 9
4.4
TROUBLE-SHOOTING ... 9
5
ARRANGING A SERVICE ... 10
6
WARRANTY INFORMATION ... 11
7
PHYSICAL DESCRIPTION... 12 7.1
DESCRIPTION OF DEVICE ... 12
7.2
TECHNICAL SPECIFICATIONS ... 12
7.2.1
Dimensions, Weight & Capacity ... 12
7.2.2
Electrical Safety ... 12
7.2.3
Electrical Supply... 12
7.2.4
Operating Conditions ... 12
7.2.5
Transport & Storage Conditions ... 12
7.2.6
Type Label ... 13
7.2.7
Key to Symbols ... 13
Page 2 of 13 IFUT688 V4 March 2013
FRESENIUS MEDICAL CARE
1
MEDICAL TREATMENT CHAIR
INSTRUCTION MANUAL - T688 Series
INTRODUCTION, PURPOSE & GENERAL SAFETY ISSUES The T688 series Medical Treatment Chair has been designed and manufactured with the intention of being used for medical procedures. These include blood collection, renal dialysis, chemotherapy and similar procedures that require comfortable and safe seating. The T688 shall only be used indoors on flat surfaces The patient should, at all times, be under the supervision of trained healthcare professionals who have been instructed by the manufacturer in the safe operation of the chair. The chair should never be used in a manner for which it was not intended. The chair is designed so that the occupant will be seated in a typical seated position - hips moved back so that the occupant’s spine is against the backrest, with legs outstretched and supported by the seat and leg rest. The weight of the occupant must not exceed 200kg for the T688 model. The chair is fitted with castors, which are specifically used to aid cleaning and/or positioning of the chair within the room. The T688 shall not be used to transport patients or any other items. Ensure that the backrest is fully elevated before moving the chair. For models fitted with dual hand controls: the programmable unit is for the use of the staff members only, and the dual recline unit, where fitted, is to be used by the patient. This ensures that the chair is not raised or lowered, by the occupant. A staff member should ensure the area surrounding the chair is free from obstruction before adjusting it. It is the staff member's responsibility to ensure that prior to operating the chair, the surrounding walls or equipment will not come into contact with nor obstruct the free movement of the chair. It is the staff members' responsibility to ensure the patient is briefed on the safe operation of the chair. If the staff member believes the patient cannot safely operate the chair, the staff member should remove the hand control from the patient’s reach. In an emergency, the chair can be lowered to the Trendelenburg position where CPR may be performed. It is the staff members' responsibility to ensure that the chair is in the correct position for CPR and that the resuscitation method used is in line with hospital policy. In addition, staff should ensure that the chair is adequate for those resuscitation methods, in line with hospital policy. In general, no responsibility or liability can be accepted by the manufacturer for failure to adhere to the guidelines and instructions contained in this manual.
Page 3 of 13 IFUT688 V4 March 2013
FRESENIUS MEDICAL CARE
2
MEDICAL TREATMENT CHAIR
INSTRUCTION MANUAL - T688 Series
OPERATING THE MEDICAL TREATMENT CHAIR 1. When the chair is stationary and positioned to give minimum interference to staff, ensure that all four brakes are locked by depressing the pedal on top of each castor. 2. The seat height may be raised to minimize bending during procedures. It is normal to cannulate whilst in this position, ensuring the patient’s legs are elevated. 3. The swing out arm is released by pushing the red latch (located below the side upholstery) forward. 4. The gas spring-assisted adjustable height armrests (if fitted) are positioned by loosening the lever and allowing the armrest to comfortably support the patient’s arm. When in the desired position, retighten the lever. 5. The swivel armrest (if fitted) is controlled by loosening the knob under the arm, rotating the arm to the desired position and retightening the knob. 6. Fold out tray arm (if fitted) is accessed by opening the upper section outwards. 7. Arms maybe swung out or lowered flat (depending on style) to seat level to facilitate side transfer of patient. Raise or lower the seat height to prevent unnecessary bending or lifting by staff members. 8. When the optional patient hand control is fitted, controls of the seat/footrest and backrest positions are available to the patient. Allow the patient to position themselves using the 4 buttons to achieve maximum personal comfort, and to regularly alter this position. 9. For safety, only nursing staff should operate the HI/LO function. The chair is supplied with the following preset positions 10. In an EMERGENCY, press memory position #1 and the backrest and leg rest will go to their flattest position and the chair will lower until the backrest is supported by the rear support bar. CPR may be performed in this position. NOTE: It is not intended that these instructions override any Hospital instructions for emergency treatment. 11. To allow the patient to rise from the chair, lower the chair and return it to the upright position. (press memory position #2) 12. Memory position #3 will be pre-set to a position suitable for renal dialysis, blood collection or similar procedures, for ICU and emergency wards, or to a position suitable for side transfer. 13. To reprogram the pre-set positions see Section: 3.3.2 14. Ensure procedural equipment is set up to avoid tubing and cords becoming trapped in the moving parts of the chair (e.g. arm rests). DISCONNECT procedural equipment prior to removing patient from chair. 15. The power cord should always be left in the power supply socket and switched on. The backup batteries (if fitted) will recharge automatically and slow to trickle charge when full.
Page 4 of 13 IFUT688 V4 March 2013
FRESENIUS MEDICAL CARE
3
MEDICAL TREATMENT CHAIR
INSTRUCTION MANUAL - T688 Series
INSTALLING THE MEDICAL TREATMENT CHAIR Note: Transport damage, if any, should be inspected and reported immediately after delivery. No claims for transport damage will be accepted 7 days after the delivery date.
At all times, when positioning the chair, care should be taken to ensure that no part of the chair comes into contact with any equipment or structures, particularly during emergency procedures. It is important to ensure that any staff members who are operating the chair have been trained by the manufacturer or its agent prior to use. The installation procedure is as follows: 1. Position the chair. 2. Remove any temporary ties or packaging. 3. Plug the power cord to an approved power supply for the relevant country as indicated by the label. 4. Test the chair by taking it through its complete range of movements. The power cord should be left in the power supply and switched on (Refer to section 3.2). The backup batteries (if fitted) will recharge automatically and slow to trickle charge when full. The chair can be operated in the normal manner immediately after commissioning.
3.1
WARNINGS AND PRECAUTIONS
The following table shows potential hazards that have been identified and the steps that should be taken to avoid them: IDENTIFIED HAZARD
CAUSE
Cushion support frame wear and tear
Operator handling error or misuse. Incorrect cleaning of upholstery
Chair stops responding to controls
Power cord damage, motor stops working, controls stop working.
Contamination on upholstery
Chair not cleaned effectively between treatments, damaged upholstery.
Instruction manual not read or understood.
Staff turnover, new staff.
PREVENTATIVE ACTION • • •
Service regularly. Train all operators. Check chairs regularly for upholstery cuts, cracks or damage. Inspect the frame for deformities or cracks
• • • •
Don’t push chair over power cords. Don’t place stress on power cords. Only use approved parts and motors. Use only as directed in this instruction manual.
• •
Take care not to damage upholstery. Damaged upholstery should be repaired or replaced immediately. Clean and decontaminate chair with appropriate agents and procedures as described in this instruction manual. Section 4.2
•
• •
Provide training for all new staff using the chair. We recommend that the operation manual stays with the chair.
Page 5 of 13 IFUT688 V4 March 2013
FRESENIUS MEDICAL CARE
3.2
MEDICAL TREATMENT CHAIR
INSTRUCTION MANUAL - T688 Series
BATTERY BACKUP (if fitted)
The battery should only be used in the event of no power supply and should not be the main source of power. As such, the power cord should be left in the power supply and switched on while the chair is in use. The battery must be charged for at least 12 hours before it can be used. The battery will automatically trickle charge until fully charged and should not be unplugged. Should the battery fail to charge, please contact Fresenius Medical Care or your local representative at the details provided in section 5. The battery is not user replaceable. The chair must be connected to the power supply to recharge at least every 3 days.
3.3
HAND CONTROLS & PROGRAMMING
Each T688 Medical Treatment Chair can be supplied with two hand controls. One nurseoperated hand control and one patient-operated hand control. The patient’s individual hand control allows operation of the seat/footrest and backrest position. Allow the patient to position themselves for maximum comfort, and to regularly alter this position. Only nursing staff should operate the HI/LO function and this control should be kept out of reach of the patient at the rear of the chair. For an explanation of symbols on the hand controls, see section 7.2.7.
3.3.1
Hand Control Patient’s Hand Control
Nurse’s Hand Control
.
Page 6 of 13 IFUT688 V4 March 2013
FRESENIUS MEDICAL CARE
3.3.2
MEDICAL TREATMENT CHAIR
INSTRUCTION MANUAL - T688 Series
Programming Instructions
This can only be done using the nurse’s hand control. A maximum of three positions may be programmed into the memory as follows: 1. Position the chair to the desired position using the up & down arrows for the backrest, whole chair and leg rest. 2. When the chair is in the desired position, hold down the S (store) button for five seconds until beep sounds. 3. Once the beep is sounding, select one of the numbers, 1, 2 or 3 and press it within two seconds to store the pre-programmed position. Note 1: For emergency stop, press any button (only for chairs with walk away feature fitted).
3.3.3
Recalibration
If there is a loss of feedback from any of the actuators, a ‘position lost’ beep will be heard from the control box when any button is pressed. Recalibrate as follows using the nurse’s hand control: 1. Press both up and down arrows of the HI/LO function simultaneously and hold until the beeping stops (approximately five seconds). NOTE: both HI and LO buttons must be activated at exactly the same time. 2. Initialise the system by retracting the actuators (press and hold the buttons) until they stop moving 3. Drive the HI/LO actuator into the fully retracted (down) position and hold button for one second after the actuators have stopped moving. 4. Drive the backrest actuator in to the fully retracted (down) position and hold button for one second after the actuators have stopped moving 5. Drive the leg rest actuator in to the fully retracted (down) position and hold button for one second after the actuators have stopped moving 6. The system can now be reprogrammed using the instructions in section 3.3.2.
Page 7 of 13 IFUT688 V4 March 2013
4
MAINTENANCE 4.1
INSPECTION & SERVICING
No part of the chair is user-serviceable. All servicing must be carried out by Fresenius Medical Care or authorised agent. Routine servicing, at least annually, is highly recommended in order to maintain the safe operation of the chair. A regular visual inspection at least once a month is recommended to ensure that each chair operates safely and as intended by the manufacturer. The battery cannot be changed by the user. If this is required please contact Fresenius Medical Care or authorised agent. No liability can be accepted by the manufacturer if the chair is operated whilst faulty and further damage occurs. Areas to be checked include: • Motors operate smoothly through cycle • No broken welds • Actuators connected properly • Upholstery not worn or torn
4.2
CLEANING
Cleaning should conform to the standards set by the hospital. All materials used in the manufacture of the chairs have been found to be suitable to be cleaned with standard hospital cleaning products, however to ensure suitability, a test should be carried out on an inconspicuous piece of material, or a sample provided by the manufacturer. The following should be used as a guideline only and no claims will be accepted for damage as a result of following these guidelines:
RECOMMENDED CLEANING PROCEDURE FOR MEDICAL TREATMENT CHAIRS The following recommended procedure should only be used if it generally conforms to Infection Control guidelines of the hospital. Step 1
Bodily fluids may carry infectious material. Ensure that protective garments and gloves are worn prior to commencing the cleaning operation.
Step 2
If required, remove the seat and backrest by gently pulling or lifting them off their clips.
Step 3
Remove as much of the spilt liquid as possible using a sponge type material with a dabbing motion.
Step 4
Make up a diluted bleach solution (e.g. Miltons) of 1 part bleach to 30 parts water.
Step 5
First test the solution on a small area of the vinyl. Then, pour onto spill area and wipe with a mopping action.
Step 6
Use a general purpose detergent cleaner with warm water on a cloth for a final clean
Step 7
Replace the seat and back rest by carefully clipping them back onto the frame.
CAUTIONARY NOTES ABOUT BLEACH: 1. Never allow bleach to mix with ammonia. If you have recently used ammonia on or near the area you are about to clean, do not use bleach. 2. Always take care when working with bleach and keep it away from children. Bleach is not only poisonous and corrosive, but it can damage clothes and materials. 3. Never use undiluted bleach Page 8 of 13 IFUT688 V4 March 2013
FRESENIUS MEDICAL CARE
4.3
MEDICAL TREATMENT CHAIR
INSTRUCTION MANUAL - T688 Series
FABRIC STANDARDS
Unless otherwise stated, standard upholstery fabrics used on these chairs conform to the following Australian Standards: - Flame Retardant tested to AS1530.3* - UV Stable - tested using Chemical Coated Fabrics and Film Test method CFFA-2* *NOTE: This is for Fresenius Medical Care standard supply fabric. Customer specific fabric may not meet these standards.
4.4
TROUBLE-SHOOTING
In the event the chair fails to operate correctly please take the following steps prior to contacting Fresenius Medical Care or its authorised agent. 1. Check the power point is active. 2. Check that the power cord is not damaged or worn. Check that the chair is plugged in and switched on correctly. 3. Ensure that the batteries have been on charge for an overnight period at a minimum. This should occur at least every 5 days. 4. Ensure there are no nicks or cuts in the hand control or actuator cables. 5. Determine what is not operating as follows: - The seat motor (vertically mounted under the seat) Depress the hand control button for the seat/footrest and the footrest should move. - The back motor (mounted behind the backrest) Depress the hand control button for the back and the back should raise or lower. - The lift column (mounted on top of the base) Depress the hand control button for the HI/LO control and the chair should raise or lower.
) of the chair. This can be found on the Type Label 6. Take note of the serial number ( attached to the chair. An example of the Type Label is in section 7.2.6. 7. If you are unsure about any aspect of the above steps, please contact Fresenius Medical Care Seating at the contact details provided on Page 10 of this manual.
Page 9 of 13 IFUT688 V4 March 2013
FRESENIUS MEDICAL CARE
5
MEDICAL TREATMENT CHAIR
INSTRUCTION MANUAL - T688 Series
ARRANGING A SERVICE In the event that the chair fails to operate correctly or to arrange a service please contact your local representative or Fresenius Medical Care Office.
) which can be found on the Type Label Note: Please quote the chair serial number ( attached to the chair. An example of the Type Label is in section 7.2.6.
CONTACT INFORMATION
FRESENIUS MEDICAL CARE (AUSTRALIA) PTY. LTD. 786 Stud Road Scoresby VICTORIA 3179 AUSTRALIA SERVICE TEL: SALES TEL: FAX: ABN:
+61 (0) 3 9780 9509 +61 (0) 3 9780 9500 +61 (0) 3 9764 8800 84 004 658 495
AGENT / DISTRIBUTOR:
<agent/distributor insert stamp here>
Page 10 of 13 IFUT688 V4 March 2013
FRESENIUS MEDICAL CARE
6
MEDICAL TREATMENT CHAIR
INSTRUCTION MANUAL - T688 Series
WARRANTY INFORMATION The following information outlines the warranty and Conditions of Sale for the Medical Treatment Chair 1. Fresenius Medical Care will repair or replace, at its discretion, any component or assembly, which exhibits failure or undue wear when subjected to normal use, and/or used in the manner which was intended at the time of sale. 2. The warranty is limited to the original purchaser at the original delivery address. 3. When sold by a reseller, this warranty covers costs at the premises of the reseller, and if required in approved situations, transport costs from the reseller to and from Melbourne, or to a third party specified by Fresenius Medical Care. 4. Use of non-recommended cleaning agents may void the warranty. 5. The T688 Series Medical Treatment Chair is covered by a 12 month warranty. 6. T688 Series Medical Treatment Chairs are not covered by warranty if used outdoors. 7. The use of unauthorised labour will void the warranty. Contact Fresenius Medical Care for approval prior to commencing work. 8. If you are unsure about any aspect of this warranty, please contact Fresenius Medical Care at the number given on Page 10 of this manual. If you need to arrange a service, please see the instructions in section 5 of this manual.
Page 11 of 13 IFUT688 V4 March 2013
FRESENIUS MEDICAL CARE
7
MEDICAL TREATMENT CHAIR
INSTRUCTION MANUAL - T688 Series
PHYSICAL DESCRIPTION 7.1
DESCRIPTION OF DEVICE
T688 Medical Treatment Chair consists of: • Electrically powered chair with - three (3) actuators - one (1) or two (2) hand controls (one nurse control & one optional patient control) - pivoting or adjustable armrests - adjustable neck rest - 4 locking castors - leg rest - removable upholstery
7.2
TECHNICAL SPECIFICATIONS
7.2.1
Dimensions, Weight & Capacity Dimensions
Weight Capacity
7.2.2
Height: 128 – 135 cm Width: 75 – 90 cm Length: 100 cm 75 kg 200 kg
depending on options depending on options
Electrical Safety Classification according to IEC 60601:1-1:1988
7.2.3
Degree of protection against electric shock
Type B
CB 6 (Control Box) Specifications Degree of protection against ingress of solids/liquids Battery Capacity Mains voltage
IPX6 1.2 AH, 24 V 120, 230V~ 50/60 Hz
Hand Control Specifications Degree of protection against ingress of solids/liquids Degree of protection against ingress of solids/liquids
IP54 (Nurse Hand Control) IP54 (Patient Hand Control)
Actuator Specifications Degree of protection against ingress of solids/liquids
IPX4
Electrical Supply Input voltage Nominal frequency Battery type Capacity Output voltage
7.2.4
Operating Conditions Temperature range
7.2.5
110 – 240 V ~ 50 – 60 Hz Lead Acid battery 1.2 Ah 24 V DC
5ºC – 40ºC
Transport & Storage Conditions No specific requirement
Page 12 of 13 IFUT688 V4 March 2013
FRESENIUS MEDICAL CARE
7.2.6
MEDICAL TREATMENT CHAIR
INSTRUCTION MANUAL - T688 Series
Type Label Fresenius Medical Care MEDICAL TREATMENT CHAIR 688Txxxxxxxx
Model: T688
Fresenius Medical Care South Asia Pacific Pty. Ltd. Level 17, 61 Lavender Street Milsons Point NSW 2061 Australia No user serviceable parts
Power Supply: 10% Duty Cycle
230 V ~ 50 Hz 3 A 2 min. ON / 18 min. OFF
For Export Only
Fresenius Medical Care MEDICAL TREATMENT CHAIR 688Txxxxxxxx
Model: T688
Fresenius Medical Care South Asia Pacific Pty. Ltd. Level 17, 61 Lavender Street Milsons Point NSW 2061 Australia No user serviceable parts
Power Supply: 10% Duty Cycle
120 V ~ 60 Hz 4 A 2 min. ON / 18 min. OFF
For Export only
7.2.7
Key to Symbols Serial Number
CE mark
Manufacturer
For indoor use only
Degree of protection against electrical shock: Type B
ATTENTION: Consult accompanying documents
Hand Control Symbols Backrest control Memory Position 1 Memory Position 2
Raise and lower the whole chair (HI/LO)
Memory Position 3
Leg rest control
Stores chair position
Moves indicated part of chair up Moves indicated part of chair down
Authorised Representative in the European Community Fresenius Medical Care Deutschland GmbH Else-Kröner-Strasse 1 D-61352 Bad Homburg Germany Telephone: ++49 (0) 6172 609-0
Page 13 of 13 IFUT688 V4 March 2013