Fresenius Medical
multiFiltrate System
multiFiltratePRO Instructions for Use sw ver 6.0 Edition 13A-2021 Dec 2021
Instructions for Use
376 Pages
Preview
Page 1
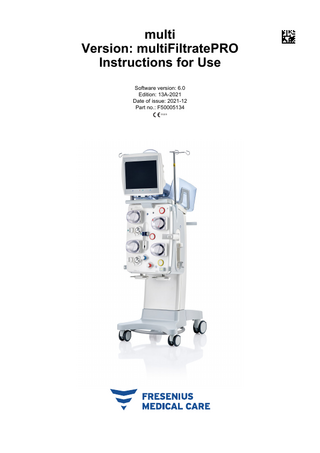
multi Version: multiFiltratePRO Instructions for Use Software version: 6.0 Edition: 13A-2021 Date of issue: 2021-12 Part no.: F50005134 0123
Table of contents 1
Index ... 13
2
Important information... 17 2.1
How to use the Instructions for Use ... 17
2.2
Significance of warnings ... 18
2.3
Significance of notes ... 18
2.4
Significance of tips... 18
2.5
Brief description ... 19
2.6 2.6.1 2.6.2 2.6.3 2.6.4 2.6.5
Intended purpose and related definitions ... 20 Intended purpose... 20 Medical indication ... 20 Intended patient population ... 20 Intended user group and intended environment... 21 Performance characteristics and clinical benefits... 21 2.6.5.1 Performance characteristics... 21 2.6.5.2 Clinical benefits ... 21
2.7 2.7.1 2.7.2
Side effects ... 23 Reporting of serious incidents ... 24 Medical Information and precautions for prevent side effects ... 24
2.8 2.8.1 2.8.2
Contraindications ... 27 Product-specific and therapy-related contraindications... 27 Relative contraindications... 28
2.9
Interaction with other systems... 29
2.10 2.10.1
Therapy restrictions ... 30 Target group ... 30
2.11
Please note the following when working on the device ... 30
2.12
Expected service life ... 31
2.13
Duties of the responsible organisation ... 31
2.14
Operator responsibility ... 32
2.15
Disclaimer of liability... 33
2.16 2.16.1 2.16.2
Warnings ... 34 Warnings about electrical safety... 34 Warnings relating to consumables and accessories ... 35
2.17
SVHC (REACH) ... 36
2.18
Addresses ... 37
Fresenius Medical Care multiFiltratePRO
IFU-EN
13A-2021
3
3
Design ... 39 3.1 3.1.1 3.1.2
4
3.1.3 3.1.4
Views of the device... 39 Front view ... 39 Rear view... 40 3.1.2.1 Connector strip ... 41 Left side view ... 42 Right side view... 43
3.2 3.2.1 3.2.2 3.2.3 3.2.4 3.2.5 3.2.6 3.2.7 3.2.8
Controls and indicators ... 44 Monitor front... 44 Monitor rear ... 45 Positioning the monitor ... 46 Using the card slot ... 47 Positioning the filter holder ... 47 Heparin pump ... 48 Heater ... 49 Extracorporeal Blood Circuit Module ... 50
3.3
User interface... 51
3.4 3.4.1 3.4.2 3.4.3
General operating concept ... 52 Colour coding on the device and single-use articles ... 52 Screen colours... 52 Context-specific information ... 53
3.5 3.5.1 3.5.2 3.5.3 3.5.4 3.5.5 3.5.6 3.5.7
Basic input procedures... 54 Changing settings with the rocker switch buttons... 54 Changing settings with the number buttons... 54 Entering data with the keyboard ... 55 On/Off button ... 56 Viewing the ratio of the UF rate to the blood flow rate... 57 Viewing the pressure values... 57 Setting the pressure alarm limit values... 59
Operation ... 61 4.1
Application principles ... 61
4.2 4.2.1 4.2.2 4.2.3 4.2.4 4.2.5
CRRT treatments ... 68 Switching on the device and starting the function test... 68 Selecting the treatment option ... 69 Continuing the previous treatment... 69 Start requirements ... 70 Mounting the cassette... 70 4.2.5.1 Mounting the return system... 71 4.2.5.2 Mounting the access system ... 72 4.2.5.3 Mounting the filtrate system ... 73 4.2.5.4 Loading the solution bags ... 73 4.2.5.5 Mounting the dialysate/substituate systems... 74 4.2.5.6 Inserting the heparin syringe ... 75 4.2.5.7 Cassette mounting completed... 76 Filling and rinsing the cassette ... 77 4.2.6.1 Filling the tubing system... 77 4.2.6.2 Entering the Patient ID and Case ID ... 77 4.2.6.3 Entering treatment parameters... 78
4.2.6
4
Fresenius Medical Care
multiFiltratePRO
IFU-EN
13A-2021
4.2.7 4.2.8 4.2.9
4.2.10 4.2.11
4.3 4.3.1 4.3.2 4.3.3 4.3.4 4.3.5
4.3.6
4.3.7 4.3.8 4.3.9
4.3.10
4.4 4.4.1
4.2.6.4 UF Rinse ... 79 Circulation... 80 Connecting the patient... 81 Treatment ... 82 4.2.9.1 Treatment screen ... 82 4.2.9.2 Menus ... 83 4.2.9.3 Histories ... 83 4.2.9.4 System Parameters... 84 Changing the treatment mode ... 84 4.2.10.1 Changing the treatment mode from CVVHDF to CVVH... 85 4.2.10.2 Changing the treatment mode from CVVHDF to CVVHD ... 86 End of treatment ... 87 4.2.11.1 Preparing the end of treatment ... 87 4.2.11.2 End of treatment with blood reinfusion... 88 4.2.11.3 Starting blood reinfusion ... 88 4.2.11.4 NaCl solution detected ... 89 4.2.11.5 Disconnecting the patient... 90 4.2.11.6 Dismantling the tubing system ... 90 CRRT Ci-Ca treatments... 91 Switching on the device and starting the function test... 91 Selecting the treatment option... 92 Continuing the previous treatment... 92 Start requirements ... 93 Mounting the cassette ... 93 4.3.5.1 Mounting the return system... 94 4.3.5.2 Mounting the access system... 95 4.3.5.3 Mounting the filtrate system ... 96 4.3.5.4 Loading the solution bags ... 96 4.3.5.5 Mounting the dialysate/substituate systems... 98 4.3.5.6 Mounting the Ci-Ca system... 99 4.3.5.7 Inserting the heparin syringe... 100 4.3.5.8 Cassette mounting completed... 102 Filling and rinsing the cassette ... 102 4.3.6.1 Filling the Ci-Ca system ... 102 4.3.6.2 Checking the Ci-Ca lines... 103 4.3.6.3 Filling the tubing system... 103 4.3.6.4 Entering the Patient ID and Case ID ... 103 4.3.6.5 Entering treatment parameters ... 105 4.3.6.6 UF Rinse ... 106 Circulation... 107 Connecting the patient... 108 Treatment ... 109 4.3.9.1 Treatment screen ... 110 4.3.9.2 Menus ... 111 4.3.9.3 Histories ... 111 4.3.9.4 System Parameters... 112 End of treatment ... 112 4.3.10.1 Preparing the end of treatment ... 112 4.3.10.2 End of treatment with blood reinfusion... 113 4.3.10.3 Starting blood reinfusion ... 113 4.3.10.4 NaCl solution detected ... 114 4.3.10.5 Disconnecting the patient... 115 4.3.10.6 Dismantling the tubing system ... 115 TPE treatments ... 117 Switching on the device and starting the function test... 117
Fresenius Medical Care multiFiltratePRO
IFU-EN
13A-2021
5
4.4.2 4.4.3 4.4.4
4.4.5
4.4.6 4.4.7 4.4.8 4.4.9 4.4.10
4.4.11
4.5 4.5.1 4.5.2 4.5.3 4.5.4 4.5.5
4.5.6
4.5.7 4.5.8
6
Selecting the treatment option ... 118 Start requirements ... 118 Mounting the cassette... 119 4.4.4.1 Mounting the return system... 119 4.4.4.2 Mounting the access system ... 121 4.4.4.3 Mounting the filtrate system ... 121 4.4.4.4 Loading the solution bags ... 122 4.4.4.5 Mounting the plasma system... 122 4.4.4.6 Inserting the heparin syringe ... 123 4.4.4.7 Cassette mounting completed... 124 Filling and rinsing the cassette ... 125 4.4.5.1 Filling the tubing system... 125 4.4.5.2 Entering the Patient ID and Case ID ... 125 4.4.5.3 Entering treatment parameters... 126 4.4.5.4 UF Rinse ... 127 Circulation... 128 Filling the plasma system ... 129 Patient connection ... 130 Preparing plasmafiltration ... 131 Treatment ... 132 4.4.10.1 Treatment screen ... 132 4.4.10.2 Menus... 133 4.4.10.3 Histories ... 133 4.4.10.4 System Parameters... 134 4.4.10.5 Performing a plasma bag change ... 134 4.4.10.6 Performing filtrate bag change (TPE)... 136 End of treatment ... 136 4.4.11.1 Preparing the end of treatment... 136 4.4.11.2 Exchanging residual plasma ... 137 4.4.11.3 Selecting blood reinfusion ... 138 4.4.11.4 End of treatment with blood reinfusion ... 139 4.4.11.5 Disconnecting the patient ... 141 4.4.11.6 Dismantling the tubing system ... 142 Paediatric CRRT treatments ... 143 Switching on the device and starting the function test... 143 Selecting the treatment option ... 144 Continuing the previous treatment... 144 Start requirements ... 145 Mounting the cassette... 146 4.5.5.1 Mounting the return system... 146 4.5.5.2 Mounting the access system ... 148 4.5.5.3 Mounting the filtrate system ... 148 4.5.5.4 Loading the solution bags ... 149 4.5.5.5 Mounting the dialysate system ... 149 4.5.5.6 Inserting the heparin syringe ... 150 4.5.5.7 Cassette mounting completed... 151 Filling and rinsing the cassette ... 152 4.5.6.1 Filling the tubing system... 152 4.5.6.2 Entering the Patient ID and Case ID ... 152 4.5.6.3 Entering treatment parameters... 153 4.5.6.4 UF Rinse ... 154 Circulation... 155 Connecting the patient when the extracorporeal blood circuit is primed with blood substitute ... 157
Fresenius Medical Care
multiFiltratePRO
IFU-EN
13A-2021
4.5.9 4.5.10
4.5.11
Connecting the patient without priming the extracorporeal blood circuit with blood substitute ... 159 Treatment ... 160 4.5.10.1 Treatment screen ... 160 4.5.10.2 Menus ... 161 4.5.10.3 Histories ... 161 4.5.10.4 System Parameters... 162 End of treatment ... 162 4.5.11.1 Preparing the end of treatment ... 162 4.5.11.2 End of treatment with blood reinfusion... 163 4.5.11.3 Starting blood reinfusion ... 164 4.5.11.4 NaCl solution detected ... 165 4.5.11.5 Disconnecting the patient... 165 4.5.11.6 Dismantling the tubing system ... 166
4.6 4.6.1 4.6.2
Treatment displays... 167 Pressure / alarm history... 167 Next operator action ... 167
4.7 4.7.1 4.7.2 4.7.3
Menus ... 168 Setting the level in the bubble catcher... 168 Cancelling preparation... 168 Treatment pause ... 168 4.7.3.1 Treatment pause with blood reinfusion (CRRT only) ... 169 4.7.3.2 Treatment pause without blood reinfusion ... 173 Switching balancing off/on... 178 Change syringe ... 179 Care mode is active... 179 Switching between predilution and postdilution... 181 Bag change (substituate/dialysate/filtrate)... 181 Ci-Ca information... 182 Ca bag change ... 183 Citrate bag change ... 184 Switching off Ci-Ca anticoagulation... 184 Switching on Ci-Ca anticoagulation... 185 Plasma volume calculation / Target volume input (TPE only) ... 187 Switching blood leak monitoring off (TPE only) ... 188
4.7.4 4.7.5 4.7.6 4.7.7 4.7.8 4.7.9 4.7.10 4.7.11 4.7.12 4.7.13 4.7.14 4.7.15 4.8 4.8.1 4.8.2 4.8.3 4.9 4.9.1
4.9.2 4.9.3
Histories ... 190 Balance data... 190 4.8.1.1 CRRT ... 190 4.8.1.2 TPE ... 192 Balance history ... 193 Events... 193 System Parameters ... 195 Access without UserCard ... 195 4.9.1.1 Pressure selection... 196 4.9.1.2 Device information ... 196 4.9.1.3 Basic settings ... 197 Access with UserCard ... 198 4.9.2.1 Applications... 198 User setup ... 200 4.9.3.1 Heparin... 200 4.9.3.2 User interface... 201 4.9.3.3 Paediatric CRRT treatments ... 202 4.9.3.4 CRRT ... 204 4.9.3.5 TPE ... 208
Fresenius Medical Care multiFiltratePRO
IFU-EN
13A-2021
7
4.10 4.10.1 4.10.2
5
6
Alarm processing ... 213 5.1
Repeated confirmation of a message ... 213
5.2 5.2.1 5.2.2
Alarm schemes ... 214 Alarm scheme one... 215 Alarm scheme two ... 215
5.3
High-priority alarm conditions ... 216
5.4
Alarm system ... 217
5.5
Response of the alarm system... 218
5.6
Messages... 220
5.7
Messages during the functional test ... 221
5.8
UF/BF message... 221
5.9
Ratio of calcium flow to filtrate flow ... 222
5.10
Ratio of citrate flow to blood flow ... 223
5.11
Ratio of plasma rate to blood flow ... 223
5.12 5.12.1 5.12.2
Pressure deviation messages ... 224 Resetting the alarm limit windows ... 224 Reducing the access pressure ... 225
5.13 5.13.1 5.13.2 5.13.3
Message “Air detected downstream of the bubble catcher”... 226 Before beginning deaeration procedures... 226 Air detected... 227 Deaeration procedures ... 227
5.14 5.14.1 5.14.2
Message “Microbubbles detected downstream of bubble catcher”... 230 Before removing the microbubbles ... 230 Microbubbles detected... 231
5.15
Blood leak... 232
5.16
Dynamic pressure test, return/insertion line ... 233
5.17 5.17.1 5.17.2
Power failure (mains power failure) ... 234 During preparation ... 234 During treatment ... 235
5.18
Display failure ... 236
5.19
Manual blood reinfusion ... 236
5.20
Manually opening the pressure measurement units... 237
Cleaning / disinfection ... 239 6.1 6.1.1
8
Network... 211 Observe before use ... 211 PDMS connection ... 212
Surface cleaning / surface disinfection ... 239 Cleaning the display ... 240
Fresenius Medical Care
multiFiltratePRO
IFU-EN
13A-2021
7
6.1.2
Detachable device components ... 240
6.2
Disinfectants and cleaning agents ... 241
Functional description ... 243 7.1
Device functions... 243
7.2 7.2.1
Description of therapies ... 243 Continuous renal replacement therapy... 243 7.2.1.1 CVVH ... 245 7.2.1.2 CVVHD... 248 7.2.1.3 CVVHDF ... 250 Therapeutic plasma exchange ... 253 Paediatric CRRT treatments... 257
7.2.2 7.2.3 7.3 7.3.1 7.3.2
8
9
Anticoagulation ... 259 Systemic anticoagulation... 259 CVVHD or postCVVHDF with the Ci-Ca protocol (regional citrate anticoagulation)... 261 7.3.2.1 Treatment prescription and essentials ... 262 7.3.2.2 Solutions for the Ci-Ca protocol ... 264 7.3.2.3 Therapy settings and monitoring with the Ci-Ca protocol ... 267 7.3.2.4 Monitoring technique and frequencies during normal operation ... 275 7.3.2.5 Unusual situations during treatment... 278
Consumables, accessories, additional equipment ... 281 8.1 8.1.1 8.1.2 8.1.3 8.1.4 8.1.5 8.1.6 8.1.7
Consumables ... 282 multiFiltratePRO Treatment kits ... 282 Haemofilters/plasma filters ... 283 Isotonic NaCl solutions ... 283 Dialysate and haemofiltration solutions ... 283 Citrate solution... 284 Disposable syringes ... 284 Other single-use items... 285
8.2
Additional equipment... 286
Installation ... 287 9.1 9.1.1 9.1.2 9.1.3
Connection requirements ... 287 Environment ... 287 Power supply network ... 287 Electrical installation ... 288
9.2
Installation / initial start-up requirements ... 288
9.3
Important information on initial start-up ... 289
Fresenius Medical Care multiFiltratePRO
IFU-EN
13A-2021
9
10 Transport / storage ... 291 10.1
Relocation ... 291
10.2
Transport ... 293
10.3 10.3.1
Storage ... 293 Storage conditions ... 294
10.4
Environmental compatibility / disposal ... 294
11 Technical Safety Checks / maintenance procedures ... 295 11.1
Important information on the Technical Safety Checks / maintenance procedures... 295
12 Specifications ... 297 12.1
Dimensions and weight... 297
12.2 12.2.1 12.2.2
Identification label (device marking)... 297 Identification label of the device... 297 Power label ... 298
12.3
Electrical safety ... 298
12.4
Electric power supply... 299
12.5 12.5.1 12.5.2
Information on electromagnetic compatibility (IEC 60601-1-2:2014)... 299 Minimum distances between radiation source and medical electrical equipment ... 300 Guidance and manufacturer's declaration on EMC ... 301
12.6
Operating conditions... 303
12.7
Storage conditions ... 304
12.8
External connection options ... 304
12.9
Operating programs ... 306
12.10
Balancing/dialysate circuit and safety systems ... 306
12.11
Extracorporeal blood circuit and safety systems... 309
12.12
Materials used... 314
13 Definitions ... 321
10
13.1
Definitions and terms ... 321
13.2
Abbreviations... 323
13.3
Symbols... 325
13.4
Certificates ... 328
Fresenius Medical Care
multiFiltratePRO
IFU-EN
13A-2021
14 Options ... 329 14.1
multiFiltratePRO-HL7-connector... 329
15 Appendix ... 347 15.1
Instructions on the use of “free software”... 347
Fresenius Medical Care multiFiltratePRO
IFU-EN
13A-2021
11
12
Fresenius Medical Care
multiFiltratePRO
IFU-EN
13A-2021
Chapter 1: Index
1
Index
A Abbreviations 323 Access pressure 309, 321 Access pressure measurement unit (red) 50 Access system 321
Blood leak/haemolysis detector 306
Context-specific information 53
Blood pump 50, 311, 321
Continuous renal replacement therapy 243
Blood reinfusion 236
Contraindications 27
Bubble catcher 168
Controls and indicators 44 Convection 322
Accessories 286
C
CRRT Ci-Ca treatments 91
Accessories case 40
Ca bag change 183
CRRT types 244
Additional equipment 286
Calcium drip counter (white) 50
CVVHD 248
Air bubble detector 50, 311
Calcium fill level detector (white) 50
D
Calcium flow 321
Deaeration procedures 227
Air detected 227 Alarm function check 321 Alarm limit 321 Alarm output 326 Alarm priorities 218 Alarm processing 213
Calcium pump (white) 50, 313, 321 Cancel preparation 72, 95, 121, 148
Definitions 321 Device functions 243 Dialysate 283, 322 Dialysate pump 50
Card for use by service engineers 323
Diffusion 322
Alarm scheme two 215
Card slot 43, 45, 321
Dimensions 297
Alarm schemes 214
Care mode is active 179
Disclaimer of liability 33
Alarm system 217
Cassette detector 50, 313
Disinfectants 241
Ambient temperature sensor 309
Certificates 328
Disinfection 239
Anticoagulation 259
Change syringe 179
Display failure 236
Appendix 347
Changing settings with the number buttons 54
Disposable syringes 284
Alarm scheme one 215
Application principles 61 Audible tone 312 Audio paused 44
B Bag change 181 Bag change (substituate/dialysate/filtrate) 181 Balance data 190 Balance history 193 Balancing 191, 243 Balancing error 191, 308 Basic input procedures 54 Battery 293, 321 Blood circuit module 50 Blood leak 233 Blood leak detector 321 Blood leak detector (yellow) 50
Changing settings with the rocker switch buttons 54
Duties of the responsible organisation 31
Ci-Ca drip counter 313
E
Ci-Ca fill level detector 313
Electrical installation 288
Circulation 306 Citrate bag change 184 Citrate dose 321 Citrate drip counter (green) 50 Citrate fill level detector (green) 50 Citrate flow 321 Citrate pump (green) 50, 313, 321
Electrical safety 298 Electromagnetic emissions 301 Electromagnetic immunity 301 Electrostatic discharge 29 End of treatment / Blood reinfusion 306
Citrate solution 284
Environmental compatibility / disposal 294
Cleaning 239
Equipotential bonding 41
Cleaning agents 241
Events 193
Cleaning the display 240
Exchange volume 322
Connection options 304
Expected service life 31
Connector strip 40, 41
External connection options 304
Consumables 282
Fresenius Medical Care multiFiltratePRO
IFU-EN
13A-2021
13
Chapter 1: Index
Extracorporeal blood circuit 243, 322
Important information on initial start-up 289
N
Extracorporeal blood circuit and safety systems 309
Important information on the Technical Safety Checks / maintenance procedures 295
Net UF volume 323
Extracorporeal Blood Circuit Module (CRRT) 50
NaCl solutions 283 Network (LAN) 326
Initial start-up 289
Next operator action 167
Initial start-up requirements 288
Note symbol, significance 18
F
Insertion switch 323
Nurse call port 41
Fill level detector 50, 311
Installation 287
Filling the tubing system 306 Filter holder 43
IV pole (left and right) 39
O On/Off button 56
Filter life 191, 322
K
Filtrate bag 322
Keyboard 55
Operating programs 306
Filtrate pressure measurement unit (yellow) 50
Kit service life 323
Operating status indicator (traffic light) 44
Filtrate pump 50
L
Operator responsibility 32
Filtration 322 Flow rates 307 Free software 347 Front view of device 39 Functional description 243 Functional test 243, 306
G
Operating conditions 303
LAN (local area network) network connection 41
Optical detector 50, 311
LAN (network) 326
P
Left side view of the device 42
Patient connection 306
Limit monitoring 57
Performing filtrate bag change (TPE) 136
Limit values 57 Line occlusion clamp (blue) 50 Line occlusion clamp (red) 50
Positioning the filter holder 47 Positioning the monitor 46
General operating concept 52
Loudspeaker 45
Post CVVH 245
H
M
Haemodialysis 322 Haemofilters/plasma filters 283
Mains power failure (power failure) 234
Post-filter calcium concentration 323
Haemofiltration 322
Maintenance procedures 295
Haemofiltration solutions 283
Manual blood reinfusion 236
Heater (green) 42
Manually opening the pressure measurement units 237
Heater (white) 42 Heater microswitch 309 Heparin pump 43, 48, 312, 322 High-priority alarm conditions 216 Histories 190 Hook-up test 322 How to use the Instructions for Use 17
I Identification 40 Identification label 40 Important information 17
14
Materials 314 Menu bar 51 Menu panel 52 Menus 168 Messages 220 Microbubbles 230 Microbubbles detected downstream of bubble catcher 230 Monitor 39, 44 Monitor arm 45 Monitor rear 45 Monitor/buttons 44
Fresenius Medical Care
Postdilution 75, 150, 323
Power failure (mains power failure) 234 Power label 40 Power supply connection 41 Power switch 41 Pre CVVH 245 Predilution 74, 150, 323 Predilution substituate pump 50 Pre-filter pressure 310 Pre-filter pressure measurement unit (red) 50 Preparation 306 Preparation time 323 Pressure / alarm history 167 Pressure alarm windows 59 Pressure displays 51 Pressure measurement units 41 Pressure values 57
multiFiltratePRO
IFU-EN
13A-2021
Chapter 1: Index
Progress bar 51
R Ratio of calcium flow to filtrate flow 222 Ratio of citrate flow to blood flow 223 Rear view of device 40 Recessed grip 45 Recommended separation distances 303 Regional citrate anticoagulation 261 Relocation 291
Switching balancing off/on 178
Ultrafiltration 307
Switching off Ci-Ca anticoagulation 184
User interface 51
Switching on Ci-Ca anticoagulation 185
Using the card slot 47
Symbols 325
V
System Parameters 195, 306
UserCard 321, 323
Systemic anticoagulation 259
Vascular access 244
Systemic calcium concentration 323
W Warning about electrical safety 34
T
Warning symbol, significance 18
Target group 30
Warnings relating to consumables and accessories 35
Technical Safety Checks / maintenance procedures 295
Warnings, electrical 34 Weight 297
Return pressure 309, 323
Temperature 79, 106, 127, 154 Terms 321
Wheels with brakes 42
Return pressure sensor (blue) 50
Therapies, description 243
Return system 323
Therapy restrictions 30
Resetting the alarm limit windows 224
Right side view of the device 43
Tip symbol, significance 18
Rinsing 306
TMP 310
Rocker switch buttons 52
Touchscreen panel 44
RS 232 port 41
Transport 293
S
Treatment data, CVVH 247
Scale 1 (green) 40
Treatment data, CVVHD 250
Scale 2 (white) 40
Treatment data, CVVHDF 253
Scale system 308
Treatment data, Pre-post CVVH 248
Scales 39 Screen colours 52 Screen failure sensor 44 Service port 41 ServiceCard 321, 323 Setting the level in the bubble catcher 168 Setting the pressure alarm limit values 59 Side effects 23 Specifications 297 Status bar 51 Storage 293 Storage conditions 294 Substituate 323 Substituate pump 50 Surface cleaning / surface disinfection 239
Treatment 306
Treatment data, TPE 256 Treatment displays 167 Treatment pause 306 Treatment pause (circulation with NaCl solution) 174 Treatment pause (circulation with recirculation connector) 175 Treatment pause without blood reinfusion 173 Treatment therapies and fields of application 23 Treatment time 191, 323 Trolley with brakes 39
U UF rate 57 UF/BF 221
Fresenius Medical Care multiFiltratePRO
IFU-EN
13A-2021
15
Chapter 1: Index
16
Fresenius Medical Care
multiFiltratePRO
IFU-EN
13A-2021
Chapter 2: Important information
2
Important information
2.1
How to use the Instructions for Use Device type
In this document, unless otherwise stated, the word “device” on its own always refers to the multiFiltratePRO device.
Identification
The document can be identified by the following information on the title page and on the labels, if any: – Software version of the device – Edition of the document – Date of issue of the document – Part number of the document
Footer
The footer contains the following information: – Company name – Device type – The English abbreviation for the document type and the international abbreviation for the document language, e.g., IFU-EN means Instructions for Use in English. – The edition identification, for example, 13A-2020 refers to edition 13A released in 2020. – The page identification
Organisation of the chapters
To facilitate the use of documents from Fresenius Medical Care, the organisation of the chapters has been standardised in all manuals. There may therefore be chapters within this document without any content. Chapters without content are marked accordingly.
Styles used in the document
The following text styles may be used in the document: Style
Description
Keys and buttons
Keys and buttons on the device are shown in bold type. Example: OK button
Display messages
Device messages are shown in bold type. Example: Message: Mains power failure
Instructions
Instructions are indicated by an arrow . Instructions must be followed. Example: Press the OK button to apply the displayed data.
Illustrations
Fresenius Medical Care multiFiltratePRO
The illustrations used in the documents may differ from the original if this does not have any influence on the function.
IFU-EN
13A-2021
17
Chapter 2: Important information
Importance of the instructions
The Instructions for Use are part of the accompanying documents and are an essential part of the device. They include all the necessary information for operating the device. The Instructions for Use must be carefully studied before attempting to operate the device.
2.2
Changes
Changes to documents will be released as new editions or supplements. In general, this manual is subject to change without notice.
Reproduction
Reproduction, even in part, is only permitted with written approval.
Significance of warnings Advises the operator of hazards that carry the risk of serious to potentially life-threatening bodily injury to persons, unless the measures for avoiding the risk described are followed. Warning Type of hazard and risk Possible consequences of exposure to the risk. Measures for avoiding the risk. Warnings can deviate from the above template in the following cases: – If a warning describes several risks – If no specific risks can be detailed in the warning
2.3
Significance of notes Note Advises the operator that the following effects can be expected in the event of failure to observe this information: – Damage to the device. – Required functions failing to run at all or running incorrectly.
2.4
Significance of tips Tip Information providing useful tips for easy handling.
18
Fresenius Medical Care
multiFiltratePRO
IFU-EN
13A-2021
Chapter 2: Important information
2.5
Brief description The device enables extracorporeal blood purification procedures to be performed. It controls and monitors the extracorporeal blood circuit. There are four operating buttons on the monitor. Input of treatment parameters and operator control is effected mostly by way of a high-resolution touchscreen. While treatment is in progress, the treatment parameters are displayed. Tube pumps are used to convey the blood, filtrate, dialysate, substituate or blood plasma, as well as the citrate and calcium solutions if citrate anticoagulation is used, depending on the procedure. For volume replacement therapies, balancing is gravity-controlled using scales, while integrated heaters can be used to heat the dialysate, substituate or replacement plasma as necessary, depending on the treatment mode. In the extracorporeal blood circuit, the blood is passed through a filter or an adsorber. The blood can be continuously anticoagulated. An air bubble detector prevents the infusion of air to the patient. Any dangerous loss of blood is prevented by a blood leak detector and by monitoring the return pressure. The access pressure monitoring unit can detect an occlusion of the needle or catheter, e.g. due to suction to the vessel wall.
Fresenius Medical Care multiFiltratePRO
IFU-EN
13A-2021
19
Chapter 2: Important information
2.6
Intended purpose and related definitions
2.6.1
Intended purpose Control, operation and monitoring of extracorporeal treatment.
2.6.2
Medical indication – Acute renal insufficiencies requiring continuous renal replacement therapy (CRRT). – Volume overloads requiring continuous renal replacement therapy (CRRT). – Certain intoxications requiring continuous renal replacement therapy (CRRT). – Diseases requiring the exchange of blood plasma by TPE. – Diseases requiring CRRT combined with haemoperfusion in order to remove additional pathogens from the blood. – Diseases requiring CRRT combined with ECCO2R for the purposes of additional CO2 removal. – Diseases requiring CRRT in addition to extracorporeal gas exchange (oxygenation and decarboxylation) intended to provide extracorporeal cardiac and/or pulmonary assist.
2.6.3
Intended patient population CVVHD, CVVHDF and CVVH treatments in adult mode are to be used in all patients requiring CRRT without or with systemic anticoagulation and with a body weight of 40 kg and more, irrespective of their age. Ci-Ca CVVHD and Ci-Ca post-CVVHDF treatments are to be used in adult patients requiring CRRT-RCA, with a body weight of 40 kg and more. CVVHD treatments in paediatric mode are to be used in all patients requiring CRRT without or with systemic anticoagulation with a body weight of 8 to 40 kg, irrespective of their age. TPE treatment is to be used in patients with a body weight of 40 kg and more, irrespective of their age. The combined CRRT + ECCO2R treatment is to be used in adult patients with a body weight of 40 kg and more. In addition, the patient restrictions defined in the relevant Instructions for Use must also be taken into consideration. The combined CRRT + haemoperfusion (Cytosorb or Seraph) treatment is to be used in adult patients with a body weight of 40 kg and more. In addition, the patient restrictions defined in the relevant Instructions for Use must also be taken into consideration.
20
Fresenius Medical Care
multiFiltratePRO
IFU-EN
13A-2021
Chapter 2: Important information
The combined CRRT + ECMO (with iLA membrane ventilator/iLA activve iLA kit (IPS) equipped with a CRRT connector) treatment is to be used in adult patients with a body weight of 40 kg and more. In addition, the patient restrictions defined in the relevant Instructions for Use must also be taken into consideration. There is no data available on the use of the device in pregnant or breastfeeding women. The device must not be used during pregnancy and breastfeeding unless the clinical condition of the woman requires treatment with the device.
2.6.4
Intended user group and intended environment The device must only be installed, operated and used by individuals with the appropriate training, knowledge and experience, and who are certified to have been trained. The device enables treatment in intensive care units or under similar conditions, where it must be used with close medical supervision and continuous monitoring for the applied treatment.
2.6.5
Performance characteristics and clinical benefits
2.6.5.1
Performance characteristics See the functional treatment description in Chapter 7 for details of the performance characteristics.
2.6.5.2
Clinical benefits Specific to CRRT
Clinical benefits of the CRRT treatment in critically ill patients with acute renal insufficiency, fluid overload or intoxications may include improved morbidity and survival outcomes by gentle control of fluid. In addition, acid-base and electrolyte balance as therapy can be timewise stretched up to continuous application, limiting the rate of changes in the patient (haemodynamic stability with slow fluid status changes and lower risk of cerebral oedema with slow osmotic pressure changes). The combined use of CRRT within the ECMO circuit provides the patients the benefit of both treatment modes using one extracorporeal access. The combined circuit does not change the clinical benefit of CRRT (fluid management, treatment of acute renal insufficiencies and/or intoxications in critically ill patients) or of ECMO. Clinical benefit of ECMO is dependent on the iLA device equipped with CRRT connector.
Specific to the combination therapy with haemoperfusion
Fresenius Medical Care multiFiltratePRO
The clinical benefits of haemoperfusion depend on the adsorber used.
IFU-EN
13A-2021
21