GE Healthcare
Voluson E6, E8 and E10 Instructions for Use Rev 4 Dec 2019
Instructions for Use
486 Pages
Preview
Page 1
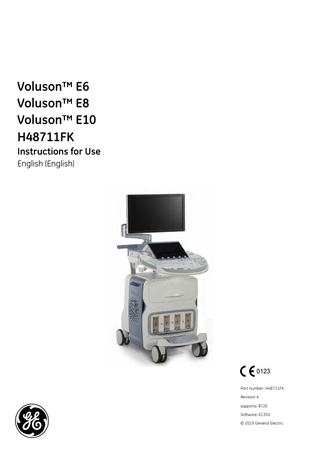
Voluson™ E6 Voluson™ E8 Voluson™ E10 H48711FK Instructions for Use English (English)
Part number: H48711FK Revision 4 supports: BT20 Software: EC350 © 2019 General Electric
Revision History
Revision
i-ii
Date
Revision 1
January 2019
Revision 2
May 2019
Revision 3
August 2019
Revision 4
December 2019
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
These Instructions for Use refer to the following Brand & Models:
Brand & Model
System
Voluson E BT19 to BT20 UPG
Voluson™ E6 / Voluson™ E8 / Voluson™ E10
Voluson E10 BT20
Voluson™ E10
Voluson E8 BT20
Voluson™ E8
Voluson E6 BT20
Voluson™ E6
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
i-iii
Table of Contents Chapter 1 – Introduction About this system - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-4 Conformance Statement - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-6 Contacting GE - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-7
Chapter 2 – Safety Symbols and Labels - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-2 Information for safe use - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-10 Electric installation - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-13 Environmental conditions for operation - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-14 Transport position - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-15 Moving the system - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-16 Operating position - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-17 Operation safety - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-18 Cleaning and disinfection of the system - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-20 Maintenance - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-23 Disposal - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-24 Bioeffects and Safety of Ultrasound Scans - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-25 Guidance and manufacturer´s declaration - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-28 Network disclosure - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-31 Cybersecurity Note - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-34 Service Software – Remote Access - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-36 Software upgrade - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-37 System messages - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-38
Chapter 3 – System description Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-2 The system - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-3 The user interface - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-5 The monitor - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-12
Chapter 4 – Getting started Powering the system - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-2 Getting started - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-3 Basic operations - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-6
Chapter 5 – Probes and Biopsies Probe safety - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-2 Cleaning and maintenance of probes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-6 Probes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-19 Biopsies - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-25 Overview of all probes and biopsies - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-31
Chapter 6 – 2D Mode 2D Mode screen display* - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-2 2D Mode standard features and modes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-4 2D Mode options - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-27
Chapter 7 – Image management TGC Slider Menu - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7-2 Scan Assistant - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7-4 Image Annotation - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7-7
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
i-iv
Table of Contents Cine Mode - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7-14
Chapter 8 – 3D and 4D Mode Visualization - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-3 General advice to obtain good rendered 3D/4D images - - - - - - - - - - - - - - - - - - - - - - - 8-7 Initial Condition of different Probes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-8 3D/4D Mode screen display* - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-10 Volume Acquisition Modes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-13 Volume Visualization Modes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-19 Additional tools - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-47
Chapter 9 – Archive Open Archive - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9-3 Data Transfer - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9-8 Source - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9-14 Patient ID - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9-18 Clipboard - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9-23
Chapter 10 – Measurements and Calculations Measurement Menu - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 10-3 Generic Measurements - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 10-5 Additional Measure Tools - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 10-16 Calculations - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 10-19 Worksheet/Report - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 10-29
Chapter 11 – Utilities and System Setup Utilities - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 11-2 System setup - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 11-7
Chapter 12 – Peripheral Devices How to Connect Auxiliary Devices Safely - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 12-2 Peripherals and hardware - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 12-5 Connection between Internal I/O and External I/O - - - - - - - - - - - - - - - - - - - - - - - - - - 12-6 DVD/USB/SW-DVR - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 12-9 ECG Preamplifier - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 12-13
Chapter 13 – Technical Data/ Information Safety conformance - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-2 Physical Attributes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-4 System overview* - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-6 Screen Formats - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-8 Display Modes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-9 Display Annotation - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-10 System Standard Features - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-13 System Options - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-15 System Parameters - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-17 Scanning Parameters - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-22 Generic Measurements and Measurements/Calculations - - - - - - - - - - - - - - - - - - - - 13-33 External Inputs and Outputs - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-39
Chapter 14 – Glossary - Abbreviations Chapter 15 – Country-specific Information
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
i-v
This page was intentionally left blank.
i-vi
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
Chapter 1 Introduction About this system - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-4 Conformance Statement - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-6 Contacting GE - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-7
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
1-1
Introduction Description of the system The Voluson™ Expert Series system is a professional diagnostic Ultrasound System which transmits Ultrasound waves into body tissues and forms images from the information contained within the received echoes. The Voluson™ Expert Series Console and related Probes are an Active Diagnostic Medical Product belonging to Class IIa according to the MDD 93/42/EEC regulation for use on human patients. The Voluson™ Expert Series Console and related Probes are an Active Diagnostic Medical Product belonging to Class IIa according to the MDR - REGULATION (EU) 2017/745 for use on human patients. The Voluson™ Expert Series system is developed and produced by GE Healthcare Austria GmbH & Co OG .
Contacting the manufacturer GE Healthcare Austria GmbH & Co OG Address
Tiefenbach 15 4871 Zipf Austria
Telephone
+43-7682-3800-0
Fax.
+43-7682-3800-47
Internet
http://www.gehealthcare.com
Diagnostic ultrasound Dear valuable Customer, We herewith would like to inform you that the American Institute of Ultrasound in Medicine (AIUM) advocates the responsible use of diagnostic ultrasound. The AIUM strongly discourages the non-medical use of ultrasound for psychosocial or entertainment purposes. The use of either two-dimensional (2D) or three-dimensional (3D) ultrasound to only view the fetus, obtain a picture of the fetus or determine the fetal gender without a medical indication is inappropriate and contrary to responsible medical practice. Although the general use of ultrasound for medical diagnosis is considered safe, ultrasound energy has the potential to produce biological effects. Ultrasound bioeffects may result from scanning for a prolonged period, inappropriate use of color or pulsed Doppler ultrasound without a medical indication, or excessive thermal or mechanical index settings (American Institute of Ultrasound in Medicine: Keepsake Fetal Imaging; 2005).Thus ultrasound should be used in a prudent manner to provide medical benefit to the patient.
About these Instructions for Use These Instructions for Use contain information on modes, features, probes and options available on the Voluson™ Expert Series system (refers to Voluson™ E6/Voluson™ E8/ Voluson™ E10). Your individual configuration may vary depending on the product you have and the country you are in. Features marked with “*” may not be available on all Voluson™ Expert Series systems. For more information see 'Overview options' on page 13-15
1-2
•
Read and understand all instructions in the Instructions for Use before attempting to use the Voluson™ Expert Series system .
• •
Keep these Instructions for Use with the product for future reference.
•
Please note that the configuration of each system is based on the specific customer order and may not contain all features listed in these Instructions for Use .
•
Some probes, options or features may not be available in some countries.
The screen graphics and illustrations in these Instructions for Use are for illustrative purposes only and may be different from what is displayed on the screen or device.
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
Introduction
•
Some features are only available on specific ultrasound consoles. Some scan modes are only available for specific ultrasound probes.
•
All references to standards / regulations and their revisions are valid for the time of publication of these Instructions for Use .
•
Paper Copy: The EU Commission Regulation on electronic instructions for use of medical devices in the European Union demands, that a paper copy of Instructions for Use can be ordered at no additional charge. You may therefore send a request to [email protected]. This request will be treated within 7 days.
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
1-3
Introduction
1.1 About this system Intended use This system is intended for use by a qualified physician or sonographer for ultrasound evaluation in the following clinical application: Image acquisition for diagnostic purposes including measurements on acquired image.
Clinical benefit The clinical benefit of a diagnostic ultrasound device is to help healthcare professionals provide an accurate diagnostic information (visualize human tissue/internal structure) that enhances the diagnostic and treatment care pathways of the patient for a variety of diseases and conditions.
Clinical applications
• • • • • • • • • •
Abdomen
• • • • •
Age: all ages (incl. embryos and fetuses)
Obstetrics (incl. Fetal Cardio) Gynecology Cardiology Transrectal Vascular Cephalic Pediatrics MSK Small Parts (incl. Breast)
Patient population Location: worldwide Sex: male and female Weight: all weight categories Height: no limitations
Do not cross-use the ultrasound system between human use and veterinary/animal use. Note
An optional veterinary/animal use kit is available.
Operator profile
• •
Qualified physicians or sonographers with at least basic ultrasound knowledge. The operator must have read and understood the Instructions for Use .
Contraindications The Voluson™ Expert Series system is not intended for:
•
1-4
ophthalmic use or any use where the probe is directly applied to the eye.
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
Introduction
• • •
trans-oesophageal use
• •
intravascular use
transuretheral use intra-operative use that is defined as introducing the ultrasound probe into a surgical incision or burr hole.
laparoscopic use
The feature "Shear Elasto" of the Voluson™ Expert Series system is not intended for:
• •
use on pregnant patients obstetrical use
Essential performance of the ultrasound system
• • • •
Acquisition of ultrasound images Display of ultrasound images on main display Measurement on ultrasound images System must remain in a safe condition acc. IEC60601
USA-FDA Indication for Use Statement The device is a general purpose ultrasound system. Specific clinical applications remain the same as previously cleared: Fetal/OB; Abdominal (including GYN, pelvic and infertility monitoring/follicle development); Pediatric; Small Organ (breast, testes, thyroid etc.); Neonatal and Adult Cephalic; Cardiac (adult and pediatric); Musculo-skeletal Conventional and Superficial; Peripheral Vascular; Transvaginal (including GYN); Transrectal
Regulatory remarks
• • •
First CE marked in 2007 (Voluson™ E6), 2006 (Voluson™ E8), 2014 (Voluson™ E10)
•
The equipment conforms with regulations for electrical safety IEC 60601 and safety class IIa according to the MDD 93/42/EEC regulation for use on human patients.
•
The equipment conforms with regulations for electrical safety IEC 60601 and safety class IIa according to the MDR - REGULATION (EU) 2017/745 for use on human patients.
Federal law restricts this device to sale by or on the order of a physician. This machine must be used in compliance with the law. Some jurisdictions restrict certain uses such as gender determination, contrast imaging, IVF, PUBS or CVS, etc. Please consider the local laws and regulations.
The manufacturer, assembler, importer or installer consider themselves responsible regarding safety, reliability and performance of the equipment under the following conditions:
• • • • •
Authorized personnel has performed installation and initial start-up of the system. Options or new settings have only been added by authorized personnel. Authorized personnel has performed modifications or repairs. The local electric installation complies with the national regulations. The equipment is only used according to the Instructions for Use .
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
1-5
Introduction
1.2 Conformance Statement The Voluson™ Expert Series system has been tested for EMC and is compliant with EN 55011 group 1 class A (CISPR 11 amendment 1) and IEC 60601-1-2. This product conforms to the following standards and regulations:
• •
CE Marked to Council Directive 93/42/EEC on Medical Devices Conforms to the following standards for safety: ○
IEC* 60601-1 Electrical medical equipment
○
IEC* 60601-1-2 Electromagnetic compatibility
○
IEC* 60601-1-6 Usability
○
IEC* 62304 Software Life Cycle Processes
○
IEC* 62366 Application of usability engineering to medical devices
○
IEC* 60601-2-37 Particular requirements for the safety of ultrasound medical diagnostic and monitoring equipment
○
ISO 10993 Biological evaluation of medical devices
○
IEC 62359 Ultrasonics - Field characterization - Test methods for the determination of thermal and mechanical indices related to medical diagnostic ultrasonic fields
○
WEEE (Waste Electrical and Electronic Equipment)
○
ROHS according to 2011/65/EU
*) Including national deviations
1-6
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
Introduction
1.3 Contacting GE For additional information or assistance, please contact your local distributor or the appropriate support resource listed on the following pages: INTERNET
http://www.gehealthcare.com http://www.gehealthcare.com/transducers
Clinical Questions
For information in the United States, Canada, Mexico and parts of the Caribbean, call the Customer Answer Center Phone: (1) 800-682-5327 or (1) 262-524-5698 In other locations, contact your local Applications, Sales or Service Representative.
Service Questions
For service in the United States, call GE CARES Phone: (1) 800-437-1171 For service for compact products in the United States, call Phone: (1) 877-800-6776 In other locations, contact your local Service Representative.
Information Request
To request the latest GE Accessories catalog or equipment brochures in the United States, call the Response Center Phone: (1) 800-643-6439 In other locations, contact your local Applications, Sales or Service Representative.
Placing an Order
To order accessories, supplies or service parts in the United States, call the GE Healthcare Technologies Contact Center Phone: (1) 800-558-5102 In other locations, contact your local Applications, Sales or Service Representative.
ARGENTINA
GEME S.A. Miranda 5237 Buenos Aires - 1407 Phone: (1) 639-1619 Fax: (1) 567-2678
ASIA PACIFIC JAPAN
GE Healthcare Asia Pacific 4-7-127, Asahigaoka Hino-shi, Tokyo 191-8503 Japan Tel: +81 42 585 5111
AUSTRALIA NEW ZEALAND
GE Healthcare Australia & New Zealand 32 Phillip Street Parramatta NSW 2150 Australia Tel: +61 2 9846 4000 8 Tangihua Street Auckland 1010 New Zealand Tel: 0800 434 325
AUSTRIA
General Electric Austria GmbH Filiale GE Healthcare Technologies EURO PLAZA, Gebäude E Wienerbergstrasse 41 A-1120 Vienna Phone: (+43) 1 97272 0 Fax: (+43) 1 97272 2222
BELGIUM &
GE Medical Systems Ultrasound Eagle Building
LUXENMBURG
Kouterveldstraat 20 1831 DIEGEM Phone: (+32) 2 719 7204 Fax: (+32) 2 719 7205
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
1-7
Introduction
BRAZIL
Av. Magalhães de Castro 4800, Andar 11 Conj. 111 e 112, Andar 12 Conj. 121 e 122, Torre 3 - Cidade Jardim São Paulo/SP - Brasil CEP: 05676-120
CANADA
GE Healthcare Ultrasound Service Engineering 9900 Innovation Drive Wauwatosa, WI 53226 Phone: (1) 800 668-0732 Customer Answer Center
CHINA
Phone: (1) 262-524-5698
GE Healthcare - Asia No. 1, Yongchang North Road Beijing Economic & Technology Development Area Beijing 100176, China Phone: (8610) 5806 8888 Fax: (8610) 6787 1162
CZECH REPUBLIC
GE Medical Systems Ultrasound Vyskocilova 1422/1a 140 28 Praha
DENMARK
GE Medical Systems Ultrasound Park Alle 295 2605 Brøndby Phone: (+45) 43 295 400 Fax: (+45) 43 295 399
ESTONIA &
GE Medical Systems
FINLAND
Kuortaneenkatu 2, 000510 Helsinki P.O.Box 330, 00031 GE Finland Phone: (+358) 10 39 48 220 Fax: (+358) 10 39 48 221
FRANCE
GE Medical Systems Ultrasound and Primary Care Diagnostics F-78457 Velizy Fax: (+33) 13 44 95 202 General Imaging: Phone: (+33) 13 449 52 43 Cardiology: Phone: (+33) 13 449 52 31
GERMANY
GE Healthcare GmbH Beethovenstrasse 239 42655 Solingen Phone: (+49) 212-28 02-0 Fax: (+49) 212-28 02 28
GREECE
GE Healthcare 8-10 Sorou Str. Marousi Athens 15125 Hellas Phone: (+30) 210 8930600 Fax: (+30) 210 9625931
1-8
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
Introduction
HUNGARY
GE Hungary Zrt. Ultrasound Division Akron u. 2 Budaors 2040 Hungary Phone: (+36) 23 410 314 Fax: (+36) 23 410 390
INDIA
Wipro GE Healthcare Pvt Ltd No. 4, Kadugodi Industrial Area Bangalore, 560067 Phone: +(91) 1-800-425-8025
ITALY
GE Medical Systems Italia spa Via Galeno, 36 20126 Milano Phone: (+39) 02 2600 1111 Fax: (+39) 02 2600 1599
KOREA
Seoul, Korea Phone: (+82) 2 6201 3114
LUXEMBOURG
Phone: 0800 2603 toll free
MEXICO
GE Sistemas Medicos de Mexico S.A. de C.V. Rio Lerma #302, 1º y 2º Pisos Colonia Cuauhtemoc 06500-Mexico, D.F. Phone: (5) 228-9600 Fax: (5) 211-4631
NETHERLANDS
GE Healthcare De Wel 18 B, 3871 MV Hoevelaken PO Box 22, 3870 CA Hoevelaken Phone: (+31) 33 254 1290 Fax: (+31) 33 254 1292
NORTHERN IRELAND
GE Healthcare Victoria Business Park 9, Westbank Road, Belfast BT3 9JL Phone: (+44) 28 90229900
NORWAY
GE Medical Systems Ultrasound Tåsenveien 71, 0873 Oslo Phone: (+47) 23 18 50 50 Strandpromenaden 45, P.O. Box 141, 3191 Horten Phone: (+47) 33 02 11 16
POLAND
GE Medical Systems Polska Sp. z o.o., ul. Wołoska 9 02-583 Warszawa, Poland Phone: (+48) 22 330 83 00 Fax: (+48) 22 330 83 83
PORTUGAL
General Electric Portuguesa SA. Avenida do Forte, n° 4 Fraccao F, 2795-502 Carnaxide Phone: (+351) 21 425 1309 Fax: (+351) 21 425 1343
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
1-9
Introduction
REPUBLIC OF IRELAND
GE Healthcare Unit F4, Centrepoint Business Park Oak Drive, Dublin 22 Phone: (+353) 1 4605500
RUSSIA
LLC GE Healthcare Presnenskaya nab., 10 123112 Moscow, Russian Federation Service center tel: 8 800 333 6967 Office Phone: (+7) 495 739 6931 Office Fax:(+7) 4957 396932
SINGAPORE
GE Healthcare Singapure 1 Maritime Square #13-012 HarbourFront Centre Singapore 099253 Tel: +65 6291 8528
SPAIN
GE Healthcare Espana C/ Gobelas 35-37 28023 Madrid Phone: (+34) 91 663 2500 Fax: (+34) 91 663 2501
SWEDEN
GE Medical Systems Ultrasound PO Box 314 17175 Stockholm Phone: (+46) 8 559 50010
SWITZERLAND
GE Medical Systems Ab Europastrasse 31 8152 Glattbrugg Phone: (+41) 1 809 92 92 Fax: (+41) 1 809 92 22
TURKEY
GE Healthcare Türkiye Istanbul Office TEL: +90 212 398 07 00 FAKS: +90 212 284 67 00 Esentepe Mah. Harman Sok. 34394 No:8 Sisli-Istanbul
UKRAINE
Authorized Representative in Ukraine Ltd. "GE Ukraine" st. Shovkovychna 42-44, m. Kyiv, 01004, Ukraine Tel: +380 44 490 69 87 Fax: +380 44 490 69 82
United Arab GE Healthcare Holding Emirates (U.A.E.) Dubai Internet City, Building No. 18 P.O. Box #11549, Dubai U.A.E. Phone: +971 4 4296161 Phone: +971 4 4296101 Fax: +971 4 4296201
1-10
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
Introduction
UNITED KINGDOM
GE Medical Systems Ultrasound 71 Great North Road Hatfield, Hertfordshire, AL9 5EN Phone: (+44) 1707 263570 Fax: (+44) 1707 260065
USA
GE Healthcare Ultrasound Service Engineering 9900 Innovation Drive Wauwatosa, WI 53226 Phone: (1) 800-437-1171 Fax: (1) 414-721-3865
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
1-11
Introduction This page was intentionally left blank.
1-12
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
Chapter 2 Safety Symbols and Labels - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-2 Information for safe use - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-10 Electric installation - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-13 Environmental conditions for operation - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-14 Transport position - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-15 Moving the system - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-16 Operating position - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-17 Operation safety - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-18 Cleaning and disinfection of the system - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-20 Maintenance - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-23 Disposal - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-24 Bioeffects and Safety of Ultrasound Scans - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-25 Guidance and manufacturer´s declaration - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-28 Network disclosure - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-31 Cybersecurity Note - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-34 Service Software – Remote Access - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-36 Software upgrade - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-37 System messages - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-38
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4
2-1
Safety
2.1 Symbols and Labels Description of all symbols and labels used on the system and in the Instructions for Use .
2.1.1 Warning labels used in the Instructions for Use Symbol
Meaning
Reference
Warning
General warning sign; IEC 60601-1; ISO 7010-W001
Indicates a hazard with a medium level of risk which, if not avoided, could result in death or serious injury.
Caution Indicates a hazard with a low level of risk which, if not avoided, could result in minor or moderate injury.
Warning/Caution: Electric Hazard Indicates the risk of injury from electric hazards.
Warning/Caution: Biological Hazard Indicates the risk of disease transmission or infections.
Warning/Caution: Explosion Hazard Indicates the risk of injury from explosion hazards.
Warning/Caution: Moving Hazard Indicates the risk of injury from moving or tipping hazards.
Warning/Caution: Mechanical Hazard Indicates the risk of injury from mechanical hazards.
Warning/Caution: Non-ionzing Hazard Indicates the risk of injury from non-ionizing radiation.
Warning/Caution: Operating LED Indicates the risk of injury from light beams entering the eye.
2-2
General warning sign IEC 60601-1; ISO 7010-W001
General warning sign, adapted to indicate electrical hazards IEC 60601-1; ISO 3864-1,
General warning sign, adapted to indicate biological hazards IEC 60601-1; ISO 3864-1,
General warning sign, adapted to indicate explosion hazards IEC 60601-1; ISO 3864-1
General warning sign, adapted to indicate moving or tipping hazards IEC 60601-1; ISO 3864-1,
General warning sign, adapted to indicate mechanical hazards IEC 60601-1; ISO 3864-1,
General warning sign, adapted to indicate non-ionizing radiation hazards IEC 60601-1; ISO 3864-1,
General warning sign, adapted to indicate light beam hazards; Do not stare at light source IEC 60601-1; ISO 3864-1; IEC 60417
Voluson™ E6 / Voluson™ E8 / Voluson™ E10 Instructions for Use H48711FK Revision 4