GE Healthcare
Voluson S10, S10 Expert and S8t Basic User Manual Rev 6 Jan 2021
Basic User Manual
400 Pages
Preview
Page 1
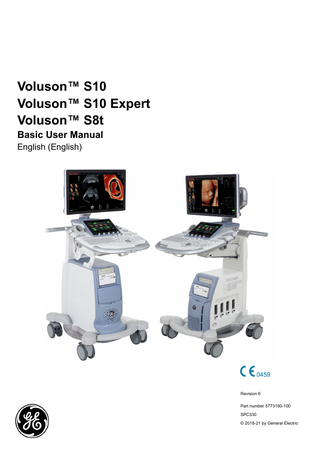
Voluson™ S10 Voluson™ S10 Expert Voluson™ S8t Basic User Manual English (English)
Revision 6 Part number 5773160-100 SPC330 © 2018-21 by General Electric
Revision History
Revision
i-ii
Date
Revision 1
January 2018
Revision 2
February 2018
Revision 3
July 2018
Revision 4
December 2018
Revision 5
January 2020
Revision 6
January 2021
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
Table of Contents Chapter 1 – General Contacting GE - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-2 Manufacturer - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-7 Safety Conformance - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-7 About this User Manual - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-8 About this system - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-9
Chapter 2 – Safety Symbols and Labels - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-2 Information for safe use - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-8 Electric installation - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-12 Environmental conditions for operation - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-12 Moving the system - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-14 Operation safety - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-15 Cleaning the system - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-16 Maintenance - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-18 Disposal - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-19 Bioeffects and Safety of Ultrasound Scans - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-19 Guidance and manufacturer´s declaration - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-22 Network disclosure - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-26 Anti-Virus Software Note - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-28 Service Software – Remote Access - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-29 Software upgrade - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-29 System messages - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-30
Chapter 3 – System description Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-2 The system - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-3 The user interface - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-4 The monitor - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-11
Chapter 4 – Getting started Powering the system - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-2 Getting started - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-3 Basic operations - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-6
Chapter 5 – Probes and Biopsies Probe safety - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-2 Cleaning and maintenance of probes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-4 Probes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-15 Biopsies - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-18 Overview of all probes and biopsies - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-23
Chapter 6 – 2D Mode 2D Mode screen display - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-2 2D Mode standard features and modes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-3 2D Mode options - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-21
Chapter 7 – Image management TGC Slider Controls - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7-2 Scan Assistant - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7-3 Image Annotation - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7-5
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
i-iii
Table of Contents
Cine Mode - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7-13
Chapter 8 – 3D and 4D Mode Visualization - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-2 General advice to obtain good rendered 3D/4D images - - - - - - - - - - - - - - - - - - - - - - - 8-7 Initial Condition of different Probes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-7 3D/4D Mode screen display - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-9 Volume Acquisition Modes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-11 Volume Visualization Modes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-17 Additional tools - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-36
Chapter 9 – Archive Open Archive - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9-2 Data Transfer - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9-7 Source - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9-12 Patient ID - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9-16 Clipboard - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9-20
Chapter 10 – Measurements and Calculations Measurement Menu - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 10-2 Generic Measurements - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 10-4 Calculations - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 10-14 Worksheet - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 10-24
Chapter 11 – Utilities and System Setup Utilities - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 11-2 System setup - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 11-8
Chapter 12 – Peripheral Devices How to Connect Auxiliary Devices Safely - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 12-2 Peripherals and hardware - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 12-3 Connection between Internal I/O and External I/O - - - - - - - - - - - - - - - - - - - - - - - - - - 12-4 DVD/USB/SW-DVR - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 12-7 ECG Preamplifier - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 12-10 Battery Pack - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 12-13
Chapter 13 – Technical Data/ Information Safety conformance - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-2 Physical Attributes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-3 System overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-6 Screen Formats - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-7 Display Modes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-8 Display Annotation - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-8 System Standard Features - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-11 System Options - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-12 System Parameters - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-13 Scanning Parameters - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-17 Generic Measurements and Measurements/Calculations - - - - - - - - - - - - - - - - - - - - 13-26 External Inputs and Outputs - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-33
Chapter 14 – Glossary- Abbreviations
i-iv
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
Chapter 1 General This chapter consists of information concerning indications for use and contact information.
Contacting GE - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-2 Manufacturer - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-7 Safety Conformance - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-7 About this User Manual - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-8 About this system - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-9
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
1-1
General
The Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t is a professional diagnostic Ultrasound System which transmits Ultrasound waves into body tissues and forms images from the information contained within the received echoes. The Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t is an Active Diagnostic Medical Product belonging to Class IIa according to the MDD 93/42/EEC regulation for use on human patients. The Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t is developed and produced by GE Ultrasound Korea, Ltd. For more Information, please contact: GE Ultrasound Korea, Ltd. Telephone
+(82) 31-740-6112
Internet
http://www.gehealthcare.com
9, Sunhwan-ro 214beon-gil, Jungwon-gu, Seongnam-si, Gyeonggi-do, Republic of Korea
• • •
First CE marked in 2016 for VOLUSON S10 First CE marked in 2018 for VOLUSON S10 Expert First CE marked in 2019 for VOLUSON S8t
Authorized EU Representative GE Medical Systems SCS 283 rue de la Miniére 78530 BUC, France Dear Valuable Customer,We here with would like to inform you that the American Institute of Ultrasound in Medicine (AIUM) advocates the responsible use of diagnostic ultrasound. The AIUM strongly discourages the non-medical use of ultrasound for psychosocial or entertainment purposes. The use of either two-dimensional (2D) or three-dimensional (3D) ultrasound to only view the fetus, obtain a picture of the fetus or determine the fetal gender without a medical indication is inappropriate and contrary to responsible medical practice. Although the general use of ultrasound for medical diagnosis is considered safe, ultrasound energy has the potential to produce biological effects. Ultrasound bioeffects may result from scanning for a prolonged period, inappropriate use of color or pulsed Doppler ultrasound without a medical indication, or excessive thermal or mechanical index settings (American Institute of Ultrasound in Medicine: Keepsake Fetal Imaging; 2005). Thus ultrasound should be used in a prudent manner to provide medical benefit to the patient.
1.1 Contacting GE For additional information or assistance, please contact your local distributor or the appropriate support resource listed on the following pages: INTERNET
http://www.gehealthcare.com http://www.gehealthcare.com/transducers
Clinical Questions
For information in the United States, Canada, Mexico and parts of the Caribbean, call the Customer Answer Center Phone: (1) 800-682-5327 or (1) 262-524-5698 In other locations, contact your local Applications, Sales or Service Representative.
Service Questions
For service in the United States, call GE CARES Phone: (1) 800-437-1171 For service for compact products in the United States, call Phone: (1) 877-800-6776 In other locations, contact your local Service Representative.
1-2
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
General
Information Request
To request the latest GE Accessories catalog or equipment brochures in the United States, call the Response Center Phone: (1) 800-643-6439 In other locations, contact your local Applications, Sales or Service Representative.
Placing an Order
To order accessories, supplies or service parts in the United States, call the GE Healthcare Technologies Contact Center Phone: (1) 800-558-5102 In other locations, contact your local Applications, Sales or Service Representative.
ARGENTINA
GEME S.A. Miranda 5237 Buenos Aires - 1407 Phone: (1) 639-1619 Fax: (1) 567-2678
ASIA PACIFIC JAPAN
GE Healthcare Asia Pacific 4-7-127, Asahigaoka Hino-shi, Tokyo 191-8503 Japan Tel: +81 42 585 5111
AUSTRALIA NEW ZEALAND
GE Healthcare Australia & New Zealand 32 Phillip Street Parramatta NSW 2150 Australia Tel: +61 2 9846 4000 8 Tangihua Street Auckland 1010 New Zealand Tel: 0800 434 325
AUSTRIA
General Electric Austria GmbH Filiale GE Healthcare Technologies EURO PLAZA, Gebäude E Wienerbergstrasse 41 A-1120 Vienna Phone: (+43) 1 97272 0 Fax: (+43) 1 97272 2222
BELGIUM &
GE Medical Systems Ultrasound Eagle Building
LUXENMBURG
Kouterveldstraat 20 1831 DIEGEM Phone: (+32) 2 719 7204 Fax: (+32) 2 719 7205
BRAZIL
GE HEALTHCARE DO BRASIL COMÉRCIO E SERVIÇOS PARA EQUIPAMENTOS MEDICOSHOSPITALARES LTDA. Av. Magalhães de Castro 4800, Andar 11 Conj. 111 e 112, Andar 12 Conj. 121 e 122, Torre 3 - Cidade Jardim São Paulo/SP - Brasil CEP: 05676-120 C.N.P.J.: 00.029.372/0001-40 Phone: 3004 2525 (Capitais e Regiões Metropolitanas)/ 0800 165 799 (Demais Localidades) Fax: (011) 3067-8280
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
1-3
General
CANADA
GE Healthcare Ultrasound Service Engineering 9900 Innovation Drive Wauwatosa, WI 53226 Phone: (1) 800 668-0732 Customer Answer Center
CHINA
Phone: (1) 262-524-5698
GE Healthcare - Asia No. 1, Yongchang North Road Beijing Economic & Technology Development Area Beijing 100176, China Phone: (8610) 5806 8888 Fax: (8610) 6787 1162
CZECH REPUBLIC
GE Medical Systems Ultrasound Vyskocilova 1422/1a 140 28 Praha
DENMARK
GE Medical Systems Ultrasound Park Alle 295 2605 Brøndby Phone: (+45) 43 295 400 Fax: (+45) 43 295 399
ESTONIA &
GE Medical Systems
FINLAND
Kuortaneenkatu 2, 000510 Helsinki P.O.Box 330, 00031 GE Finland Phone: (+358) 10 39 48 220 Fax: (+358) 10 39 48 221
FRANCE
GE Medical Systems Ultrasound and Primary Care Diagnostics F-78457 Velizy Fax: (+33) 13 44 95 202 General Imaging: Phone: (+33) 13 449 52 43 Cardiology: Phone: (+33) 13 449 52 31
GERMANY
GE Healthcare GmbH Beethovenstrasse 239 42655 Solingen Phone: (+49) 212-28 02-0 Fax: (+49) 212-28 02 28
GREECE
GE Healthcare 8-10 Sorou Str. Marousi Athens 15125 Hellas Phone: (+30) 210 8930600 Fax: (+30) 210 9625931
HUNGARY
GE Hungary Zrt. Ultrasound Division Akron u. 2 Budaors 2040 Hungary Phone: (+36) 23 410 314 Fax: (+36) 23 410 390
INDIA
Wipro GE Healthcare Pvt Ltd No. 4, Kadugodi Industrial Area Bangalore, 560067 Phone: +(91) 1-800-425-8025
1-4
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
General
ITALY
GE Medical Systems Italia spa Via Galeno, 36 20126 Milano Phone: (+39) 02 2600 1111 Fax: (+39) 02 2600 1599
KOREA
Seoul, Republic of Korea Phone: (+82) 2 6201 3114
LUXEMBOURG
Phone: 0800 2603 toll free
MEXICO
GE Sistemas Medicos de Mexico S.A. de C.V. Rio Lerma #302, 1º y 2º Pisos Colonia Cuauhtemoc 06500-Mexico, D.F. Phone: (5) 228-9600 Fax: (5) 211-4631
NETHERLANDS
GE Healthcare De Wel 18 B, 3871 MV Hoevelaken PO Box 22, 3870 CA Hoevelaken Phone: (+31) 33 254 1290 Fax: (+31) 33 254 1292
NORTHERN IRELAND
GE Healthcare Victoria Business Park 9, Westbank Road, Belfast BT3 9JL Phone: (+44) 28 90229900
NORWAY
GE Medical Systems Ultrasound Tåsenveien 71, 0873 Oslo Phone: (+47) 23 18 50 50 Strandpromenaden 45, P.O. Box 141, 3191 Horten Phone: (+47) 33 02 11 16
POLAND
GE Medical Systems Polska Sp. z o.o., ul. Wołoska 9 02-583 Warszawa, Poland Phone: (+48) 22 330 83 00 Fax: (+48) 22 330 83 83
PORTUGAL
General Electric Portuguesa SA. Avenida do Forte, n° 4 Fraccao F, 2795-502 Carnaxide Phone: (+351) 21 425 1309 Fax: (+351) 21 425 1343
REPUBLIC OF IRELAND
GE Healthcare Unit F4, Centrepoint Business Park Oak Drive, Dublin 22 Phone: (+353) 1 4605500
RUSSIA
LLC GE Healthcare Presnenskaya nab., 10 123112 Moscow, Russian Federation Service center tel: 8 800 333 6967 Office Phone: (+7) 495 739 6931 Office Fax:(+7) 4957 396932
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
1-5
General
SINGAPORE
GE Healthcare Singapure 1 Maritime Square #13-012 HarbourFront Centre Singapore 099253 Tel: +65 6291 8528
SPAIN
GE Healthcare Espana C/ Gobelas 35-37 28023 Madrid Phone: (+34) 91 663 2500 Fax: (+34) 91 663 2501
SWEDEN
GE Medical Systems Ultrasound PO Box 314 17175 Stockholm Phone: (+46) 8 559 50010
SWITZERLAND
GE Medical Systems Ab Europastrasse 31 8152 Glattbrugg Phone: (+41) 1 809 92 92 Fax: (+41) 1 809 92 22
TURKEY
GE Healthcare Türkiye Istanbul Office TEL: +90 212 398 07 00 FAKS: +90 212 284 67 00 Esentepe Mah. Harman Sok. 34394 No:8 Sisli-Istanbul
UKRAINE
Authorized Representative in Ukraine Ltd. "GE Ukraine" st. Shovkovychna 42-44, m. Kyiv, 01004, Ukraine Tel: +380 44 490 69 87 Fax: +380 44 490 69 82
United Arab GE Healthcare Holding Emirates (U.A.E.) Dubai Internet City, Building No. 18 P.O. Box #11549, Dubai U.A.E. Phone: +971 4 4296161 Phone: +971 4 4296101 Fax: +971 4 4296201 UNITED KINGDOM
GE Medical Systems Ultrasound 71 Great North Road Hatfield, Hertfordshire, AL9 5EN Phone: (+44) 1707 263570 Fax: (+44) 1707 260065
1-6
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
General
USA
GE Healthcare Ultrasound Service Engineering 9900 Innovation Drive Wauwatosa, WI 53226 Phone: (1) 800-437-1171 Fax: (1) 414-721-3865
Kazakhstan
Authorized representative in Kazakhstan: General Electric Kazakhstan LLP Timiryazev St 28V, office 307, Almaty, 050040 Kazakhstan T +7 727 3560020
1.2 Manufacturer GE Ultrasound Korea, Ltd. 9, Sunhwan-ro 214beon-gil, Jungwon-gu, Seongnam-si, Gyeonggi-do, Republic of Korea
1.3 Safety Conformance The Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t conforms to the following standards and regulations :
•
Classified to ANSIAAMI ES60601-1 2005 R1 2012 Medical Electrical Equipment, Part 1: General Requirements for Safety by a Nationally Recognized Test Lab
• • •
Certified to CSA CAN/CSA-C22.2 NO. 60601-1 :14 General requirements for safety CE Marked to Council Directive 93/42/EEC on Medical Devices Conforms to the following standards for safety: ○
IEC/EN 60601-1 Medical electrical equipment - Part1 : General requirements for safety
○
EMC Emissions Group 1. Class B device requirements as per Sub clause 5.3 of CISPR 11
○
IEC/EN 60601-1-6 Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance - Collateral Standard: Usability
○
IEC/EN 60601-2-37 Medical electrical equipment - Part 2-37: Particular requirements for the safety of ultrasonic medical diagnostic and monitoring equipment
○
IEC/EN 62366 Application of usability engineering to medical devices
○
IEC/EN 62366-1 Medical devices-Part1: Application of usability engineering to medical devices
○
IEC/EN 62304 Software Life Cycle Processes
○
IEC/EN 62359 Ultrasonic - Field characterization - Test methods for the determination of thermal and mechanical indices related to medical diagnostic ultrasonic fields.
○
EN/ISO15223-1 Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied - Part 1: General requirements
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
1-7
General
• •
○
ISO 10993-1 Biological evaluation of medical devices - Part 1 Evaluation and testing
○
WEEE ( Waste Electrical and Electronic Equipment)
○
ROHS according to 2011/65/EU
○
IEC/EN 60601-1-2 Medical electrical equipment - Part 1-2: General requirements for safety - Collateral Standard : Electromagnetic compatibility - requirements and tests
○
IEC/EN 60601-1-4 Medical electrical equipment - Part 1-4: General requirements for safety - Collateral Standard; programmable electrical medical systems
○
IEC/EN 60601-2-18 Medical electrical equipment - Part 2-18: Particular requirements for the basic safety and essential performance of endoscopic equipment
NEMA UD2 Acoustic output measurement standard for diagnostic ultrasound equipment NEMA UD3 Standard for real time display of thermal and mechanical acoustic output indices on diagnostic ultrasound equipment ( MI, TIS, TIB, TIC)
1.4 About this User Manual These Instructions for Use contain information on modes features, pobes and options available on the Voluson Signature series system (refer to Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t ) Your individual configuration may vary depending on the product you have and the country you are in.
•
Read and understand all instructions in the Basic User Manual before attempting to use the Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t .
•
This Manual has to be used in connection with the Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t .
• •
Keep this User Manual with the equipment at all times.
•
Periodically review the procedures for operation and safety precautions.
All information contained in the Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t User Manual is relevant.
Please note that orders are based on the individually agreed specifications and may not contain all features listed in this manual.
The screen graphics and illustrations in this manual are for illustrational purposes only and may be different from what you see on the screen or device
All references to standards / regulations and their revisions are valid for the time of publication of the user manual.
It might be possible that some probes, options or features are NOT available in some countries.
1-8
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
General
1.5 About this system Intended use This system is intended for use by a qualified physician or sonographer for ultrasound evaluation in the following clinical application: Image Acquisition for diagnostic purposes including measurements on acquired image.
Clinical applications
• • • • • • • • • • •
Abdomen Obstetrics (incl. Fetal Cardio) Gynecology Cardiology Transrectal Peripher Vascular Pediatrics Cephalic MSK Breast Small-parts
Patient population
• • • • •
Age: all ages (incl. embryos and fetuses) Location: worldwide Sex: male and female Weight: all weight categories Height: no limitations
Operator profile
• •
Qualified physicians or sonographers with at least basic ultrasound knowledge. The operator must have read and understood the user manual.
Contraindications The Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t system is not intended for:
• •
ophthalmic use or any use where the probe is directly applied to the eye. intra-operative use that is defined as introducing probe into a surgical incision or burr hole.
Essential performance of the ultrasound system
• • • •
Acquisition of ultrasound images Display of ultrasound images on main display Measurement on ultrasound images System must remain in a safe condition acc. IEC60601
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
1-9
General
Indications for Use Statement for US and Canada The device is a general-purpose ultrasound system. Specific clinical applications remain the same as previously cleared: Fetal/Obstetrics; Abdominal (Including renal and GYN/pelvic); Pediatric; Small Organ (breast, testes, thyroid, salivary gland, lymph nodes, pediatric and neonatal patients); Neonatal and Adult Cephalic; Cardiac (adult and pediatric); Peripheral Vascular (PV); Musculo-skeletal Conventional and Superficial; Transrectal (including Urology and Prostate) (TR); Transvaginal (TV).
Regulatory remarks
• • • •
First CE marked in 2016 (for VS10) , 2018 (for VS10 Expert) , 2019 (for VS8t) Federal law restricts this device to sale by or on the order of a physician! This machine must be used in compliance with the law. Some jurisdictions restrict certain uses such as gender determination, contrast imaging, IVF, PUBS or CVS, etc. Please consider the local laws and regulations. The equipment conforms with regulations for electrical safety IEC 60601 and safety class IIa according to the MDD 93/42/EEC regulation for use on human patients.
The manufacturer, assembler, importer or installer consider themselves responsible regarding safety, reliability and performance of the equipment under the following conditions:
• • • • • •
Authorized personnel has performed installation and initial start-up of the system. Options or new settings have only been added by authorized personnel. Authorized personnel has performed modifications or repairs. The local electric installation complies with the national regulations. The equipment is only used according to the Basic User Manual. Paper copy : The EU commission Regulation on electronic instructions for use of medical devices in the European Union demands, that a paper copy of Instructions for Use can be ordered at no additional charge. You may therefore, send a request to volusondocumentation-request@ge.com. This request will be treated within 7 days.
Importer Information
•
Türkiye İthalatçısı
GE Medical Systems Türkiye Ltd. Şti. Esentepe Mah. Harman Sok. No: 8 34394 Şişli İstanbul Türkiye
1-10
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
Chapter 2 Safety Symbols and Labels - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-2 Information for safe use - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-8 Electric installation - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-12 Environmental conditions for operation - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-12 Moving the system - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-14 Operation safety - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-15 Cleaning the system - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-16 Maintenance - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-18 Disposal - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-19 Bioeffects and Safety of Ultrasound Scans - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-19 Guidance and manufacturer´s declaration - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-22 Network disclosure - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-26 Anti-Virus Software Note - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-28 Service Software – Remote Access - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-29 Software upgrade - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-29 System messages - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-30
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
2-1
Safety
2.1 Symbols and Labels Description of all symbols and labels used on the system and in the Basic User Manual.
2.1.1 Warning labels used in the Warning Indicates a hazard with a medium level of risk which, if not avoided, could result in death or serious injury. Caution Indicates a hazard with a low level of risk which, if not avoided, could result in minor or moderate injury. Warning/Caution: Electric Hazard Indicates the risk of injury from electric hazards. Warning/Caution: Biological Hazard Indicates the risk of disease transmission or infections. Warning/Caution: Explosion Hazard Indicates the risk of injury from explosion hazards. Warning/Caution: Moving Hazard Indicates the risk of injury from moving or tipping hazards. Warning/Caution: Mechanical Hazard Indicates the risk of injury from mechanical hazards. Warning/Caution: Non-ionzing Hazard Indicates the risk of injury from non-ionizing radiation.
Warning/Caution: Operating LED Indicates the risk of injury from light beams entering the eye.
2.1.2 Description of symbols and labels Some symbols used with electrical medical equipment have been accepted as standard by IEC. They serve for marking connections, accessories, and as warnings.
2-2
Main power switch ON
IEC 60601-1:2005+ A1:2012 Annex D.1 and IEC 60417-5007
Main power switch OFF
IEC 60601-1:2005+ A1:2012 Annex D.1 and IEC 60417-5008
ECG symbol
GE created
Protective earth (ground) connection
IEC 60601-1:2005+ A1:2012 Annex D.1 and IEC 60417-5019
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
Safety
Standby button
IEC 60601-1:2005+ A1:2012 Annex D.1 and IEC 60417-5009
Insulated patient applied part (Type BF)
IEC 60601-1:2005+ A1:2012 Annex D.1 and IEC 60417-5333
Potential equilibrium connection
IEC 60601-1:2005+ A1:2012 Annex D.1 and IEC 60417-5021
Defibrillation-proof insulated patient applied part (Type CF)
IEC 60601-1:2005+ A1:2012 Annex D.1 and IEC 60417-5336
This symbol is followed by the manufacturing date of the device in the form YYYY-MM
EN ISO 15223-1:2016 and ISO 7000-2497
This symbol is followed by the name and address of the manufacturer of the device.
EN ISO 15223-1:2016 and ISO 7000-3082
This symbol is followed by the serial number of the device.
ISO 7000-2498 and EN ISO 15223-1:2016
This symbol indicates that in the United States of America, federal law restricts this device to sale by or on the order of a physician.
21 CFR 801.109 and Alternative to Certain Prescription Device Labeling Requirements Guidance to Industry 1/2/2000 U.S. Food&Drug Administration modified by General Electric for clarity that this is for the USA
Catalog or model number.
ISO 7000-2493 and EN ISO 15223-1:2016
This symbol indicates that the waste of electrical and electronic equipment must not be disposed as unsorted municipal waste and must be collected separately. Please contact the manufacturer or other authorized disposal company to decommission your equipment according to local regulations.
WEEE Diectrive 2012/19/EU
Pictogram on Probe Care Card:
GE created
Pictogram on Probe Care Card:
GE created
Use care when handling ultrasound probes and protect the probe head from damage.
Pictogram on Probe Care Card: Describes precautions necessary to prevent the risk of disease transmission or infections.
Do not immerse the probe into any liquid beyond the level specified for that probe. Refer to the user manual of the ultrasound system. IEC TR 60878-0659 EN ISO 15223-1:2016
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
Pictogram on Probe Care Card: Describes precautions necessary to prevent the risk of injury through electric hazards.
IEC 60417-6042 ISO 7010W012 IEC 60601-1:2005+ A1:2012 Annex D.2
2-3
Safety
The Common Mark of Products Circulation certifies that the products bearing this mark, passed all conformity assessment (approval) procedures established by technical regulations of the Customs Union and correspond to the requirements of all technical regulations of the Customs Union applied to these products.
Conformity with the technical regulation TR CU of the EurAsEC Customs Union
GOST-R Label
National standards of the Russian Federation and CIS countries
CE Conformity mark according to Medical Device Directive 93/42/EEC
European Directive 93/42/EEC
This product consists of devices that may contain mercury, which must be recycled or disposed of in accordance with local, state, or country laws. (Within this system, the backlight lamps in the monitor display, contain mercury.)
A chemical element with the symbol Hg and atomic number 80
Consult accompanying documents. This symbol advises the reader to consult the accompanying documents.
IEC 60601-1:2005+ A1:2012 Annex D.1 and ISO 7010-M002
Indicates that the power cable is hospital grade. Grounding reliability can only be achieved when the equipment is connected to an equivalent receptacle marked “Hospital only” or “Hospital grade”. Applicable depending on local regulatory requirements.
UL817
• 100-120V/220-240V~ :
GE created
Caution, consult accompanying documents. This symbol advises the reader to consult the accompanying documents for important safety-related information such as warnings and pre-cautions that cannot be presented on the device itself.
IEC 60601-1:2005+ A1:2012 Annex D.1, ISO 7000-0434A and EN ISO 15223-1:2016-5 .4.4
Protection against the effects IEC of immersion in water (probes) 60601-1:2005+ A1:2012 Annex D.3 and IEC 60529
No protection against ingress of water (system)
IEC 60601-1:2005_ A1:2012 Annex D.3 and IEC 60529
Indicates a USB connector.
Indicates a network connector. IEC 60417-5988
Inlet, This text indicates the voltages that the device is built for. Please note that either the first voltage range OR the second voltage range is applicable - depending on your country’s voltage. This device uses alternating current.
Green dot on power cable plug
• 900VA: Max Power Consumption
• 50/60Hz: This indicates
the electrical frequency that the device is built for. Please note that either the first frequency OR the second frequency is applicable - depending on your country’s frequency.
2-4
USB Implementers Forum, Inc
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
Safety
Product was refurbished / remanufactured by GE ULTRASOUND KOREA, LTD.
GE created
FDA Every system has a unique marking for identification. The UDI marking appears on the product labels which is located on the top of the CPU. Scan or enter the information from these labels into the patient health record as required by the governing laws.
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6
This symbol indicates ESD (electrostatic discharge) sensitivity of a connector that is not tested as specified in IEC 60601-1-2. Electrostatic discharge can damage the product. Do not touch exposed connector pins.
IEC 60417-5134
NRTL Classification Label
TUV rheinland
2-5
Safety
China This symbol indicates the product contains hazardous materials in excess of the limits established by the Chinese standard SJ/ T11364-2014 Requirements of concentration limits Electronic Industry for certain restricted substances in electrical and electronic products. Standard SJ/ The number in the symbol is the Environment-friendly Use Period (EFUP), which indicates T11364-2014 the period during which the hazardous substances contained in electrical and electronic products will not leak or mutate under normal operating conditions so that the use of such electrical and electronic products will not result in any severe environmental pollution, any bodily injury or damage to any assets. The unit of the period is “Year”. In order to maintain the declared EFUP, the product shall be operated normally according to the instructions and environmental conditions as defined in the product manual, and periodic maintenance schedules specified in Product Maintenance Procedures shall be followed strictly. Consumables or certain parts may have their own label with an EFUP value less than the product. Periodic replacement of those consumables or parts to maintain the declared EFUP shall be done in accordance with the Product Maintenance Procedures. This product must not be disposed of as unsorted municipal waste, and must be collected separately and handled properly after decommissioning. Component Name
Hazardous Substances’ Name (Pb)
(Hg)
(Cd)
(Cr(VI))
(PBB)
(PBDE)
Ultrasound Probes
X
O
O
O
O
O
LCD Monitor
X
O
O
O
O
O
Operator Panel
X
O
O
O
O
O
PWAs
X
O
O
O
O
O
Cables
X
O
O
O
O
O
Power assembly
X
O
O
O
O
O
System Covers
X
O
O
O
O
O
Frame Assembly
O
O
O
O
O
O
Rubber
O
O
O
O
O
O
This table is prepared according to SJ/T 11364. O: Indicates that hazardous substance contained in all of the homogeneous materials for this part is below the limit requirement in GB/T 26572. X: Indicates that hazardous substance contained in at least one of the homogeneous materials used for this part is above the limit requirement in GB/T 26572 .
• Data listed in the table represents best information available at the time of publication
• Applications of hazardous substances in this medical device are required to
achieve its intended clinical uses, and/or to provide better protection to human beings and/or to environment, due to lack of reasonably (economically or technically) available substitutes.
Symbol indicating that the Instructions for Use are supplied in electronic form
2-6
ISO7000-3500
Voluson™ S10 / Voluson™ S10 Expert / Voluson™ S8t Basic User Manual 5773160-100 Revision 6