User Manual
268 Pages
Preview
Page 1
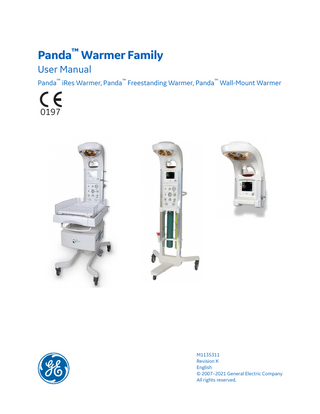
Panda™ Warmer Family User Manual Panda™ iRes Warmer, Panda™ Freestanding Warmer, Panda™ Wall-Mount Warmer
0197
M1135311 Revision K English © 2007–2021 General Electric Company All rights reserved.
User Manual
Legal notices GE Healthcare will make available on request such circuit diagrams, component diagrams, component parts lists, descriptions, calibration instructions or other information that will assist the users or appropriately qualified technical personnel to repair those parts of the equipment that are classified by GE Healthcare as repairable. General Electric Company reserves the right to make changes in specifications and features shown herein or discontinue the product described at any time without notice or obligation. Contact your GE Representative for the most current information. Panda and Giraffe are registered trademarks owned by Datex-Ohmeda, Inc. GE and GE Monogram are trademarks of General Electric Company. All other company and product names mentioned may be trademarks of the companies with which they are associated.
Masimo Possession or purchase of this device does not convey any express or implied licence to use the device with unauthorised sensors or cables that would, alone or in combination with this device, fall within the scope of one or more of the patents to this device. Masimo Patents: www.masimo.com/patents.htm
Revision history Table 1 Revision history Revision
Revision date
Description
K
2021
Warmer user manual conversion to content management system, incorporation of cleaning and disinfection information and incorporation of the addenda that follow: • Addendum, Freestanding and Wall Mount Models, 2063360-001 • Panda™ iRes and Giraffe™ Warmers Integrated SpO2 Operation and Maintenance Supplement, M1110918 • Baby Temperature Addendum, M1157982 • Panda iRes™ and Giraffe™ Warmers Resuscitation System T-piece Option Operation and Maintenance Supplement, M1109204 • Panda iRes™ and Giraffe™ Warmers Resuscitation System Bag and Mask Option Operation and Maintenance Supplement, M1110023 • Addendum Giraffe and Panda Warmer Alarms, 2080135-001 • Addendum for Union Label, 5810676 • US RoHS Addendum, M1133645 • ADDENDUM Operation and Maintenance Manual for Giraffe Warmer and Panda iRes Warmers – Bedside Panels, 5821739
J
2020
Back cover – replaced CE mark and address label location indicator box with printed CE Mark, name and address.
A-H
–
Previous revisions
M1135311 Revision K
Panda Warmer Family
3/232
Page intentionally left blank
User Manual
Contents
Contents Legal notices...3 Revision history ...3 Chapter 1 Standards and regulations ... 11 1.1 Standards ... 11 1.2 Disposal... 11 1.2.1 Patient applied parts ... 11 1.3 Electromagnetic compatibility (EMC) guidance...12 1.3.1 Electromagnetic compatibility essential performance ...12 1.3.2 Degraded performance characteristics...12 1.3.3 Guidance and manufacturer’s declaration – electromagnetic emissions...13 1.3.4 Manufacturer’s guidance and declaration regarding electromagnetic immunity ...13 1.3.5 International Electronic Commission (IEC) guidance and manufacturer’s declaration regarding electronic immunity... 14 1.3.6 Recommended separation distance between portable and mobile RF communications equipment and the system... 16 1.4 Vermont RoHS... 16 1.5 CE mark ... 16
Chapter 2 Introduction ... 17 2.1 Manual availability... 17 2.2 Intended users ... 17 2.3 User responsibility... 17 2.4 Abbreviations and acronyms ... 17 2.5 Terminology... 18 2.6 Illustrations... 18
Chapter 3 Safety ... 19 3.1 Safety definitions ... 19 3.2 Safety signs... 19 3.3 Symbols displayed on the device...20 3.4 Safety hazards... 26 3.4.1 Protection against electrical hazards...26 3.4.2 Protection against mechanical hazards...26 3.4.3 Protection regarding functional hazards ...28 3.4.4 Protection regarding equipment hazards...30 3.4.5 Protection against fire hazards ...31 3.5 Intended use ... 32
Chapter 4 References ... 33
M1135311 Revision K
Panda Warmer Family
5/232
Contents
User Manual
Chapter 5 System description... 35 5.1 Overview... 35 5.2 Features, options and models ...35 5.2.1 Freestanding and wall-mount warmer...36 5.3 Mechanical controls, cable connections and features ...38 5.4 Display and display controls... 42 5.4.1 Trends... 44
Chapter 6 Options... 45 6.1 Uninterruptible Power Supply (UPS)...45 6.2 Scales ... 45 6.3 Integrated SpO2 system ... 45 6.3.1 Identifying the SpO2 system...45 6.3.2 Nellcor SpO2 display... 46 6.3.2.1 Nellcor SpO2 system oximetry indicators...47 6.3.3 Nellcor SpO2 menu ... 49 6.3.4 Masimo SpO2 display ... 51 6.3.5 Masimo SpO2 menu... 51 6.4 Resuscitation system... 53 6.4.1 T-piece resuscitation system components...55 6.4.2 T-piece resuscitation system controls and connections ...58 6.4.3 Bag and Mask resuscitation system components...60 6.4.4 Bag and Mask resuscitation system controls and connections ...62 6.5 RS-232 (ThermaLink)... 63 6.5.1 Serial data interface... 64 6.5.2 Nurse call system interface ...64
Chapter 7 Accessories ... 65 7.1 Accessories... 65 7.2 SpO2 system accessories... 67 7.2.1 Masimo SpO2 system accessories ...67 7.2.2 Nellcor SpO2 system accessories...68 7.3 Resuscitation system accessories...69 7.3.1 T-piece resuscitation system accessories...72 7.4 ResusView™ Heart Rate option accessories ...72 7.5 Shelf accessories... 73 7.6 Mattress accessories... 73 7.7 Bedside panel accessories... 73 7.8 Dovetail rail accessories ... 76 7.9 IV pole accessories ... 80 7.10 DIN rail accessories ... 81
6/232
Panda Warmer Family
M1135311 Revision K
User Manual
Contents
7.11 UPS accessories ... 82 7.12 Phototherapy accessories... 82 7.13 Upgrades ... 83 7.14 Accessories installation... 89 7.14.1 Mounting rail system components...89
Chapter 8 Pre-use checkout and setup procedures ... 93 8.1 Mechanical checks... 93 8.2 Controller checks ... 99 8.3 Equipment checks for optional features... 101 8.3.1 Resuscitation system setup and checkout procedures ... 102 8.3.1.1 Resuscitation system gas supply connection... 102 8.3.1.2 Suction checkout... 108 8.3.1.3 T-piece resuscitation system oxygen delivery checkout ... 111 8.3.1.4 Bag and Mask resuscitation system oxygen delivery checkout... 116 8.3.2 Nurse call checkout... 118
Chapter 9 Power ON and Power OFF... 119 9.1 Startup... 119 9.2 Shutdown ... 121 9.3 Loss of power... 121
Chapter 10 Operating instructions ... 123 10.1 Adjusting the bedside panels... 125 10.2 Tilting the bed ... 129 10.3 Adjusting the bed height... 129 10.4 Putting the bassinet in position... 130 10.5 Using the X-ray tray ... 132 10.6 Using the observation and procedure lights ... 132 10.7 Home screen ... 133 10.7.1 Mode ... 133 10.7.2 APGAR ... 134 10.7.3 Menu ... 134 10.8 Setup... 135 10.9 Warm-up Mode ... 136 10.10 Control modes... 137 10.10.1 Manual Mode ... 138 10.10.2 Baby Mode ... 139 10.10.2.1 Temperature monitoring safety information... 141 10.11 Options... 142 10.11.1 Using the in-bed scale (optional) ... 142 10.11.1.1 Installing the scale... 142 10.11.1.2 Weighing procedure... 143 M1135311 Revision K
Panda Warmer Family
7/232
Contents
User Manual
10.11.2 Using the SpO2 system (optional)... 147 10.11.2.1 Nellcor oximetry overview ... 149 10.11.2.2 Masimo general description of operation ... 156 10.11.3 Using the integrated resuscitation system (optional) ... 158 10.11.3.1 Using the T-piece resuscitation system... 160 10.11.3.2 Using the Bag and Mask resuscitation system ... 163 10.11.4 Intra-hospital transport ... 164 10.11.4.1 Using the patient restraint... 165 10.11.4.2 Using the shelf restraint... 165 10.12 Attaching the skin temperature probe... 169 10.13 On screen help ... 171 10.14 Alarms... 174 10.14.1 Alarm priorities... 175 10.14.2 Multiple alarms ... 175 10.14.3 Silencing alarms ... 176 10.14.4 Setting the manual temperature alarm... 177 10.14.5 Baby Hot – Check Temp Probe... 177 10.14.6 Baby Cold - Check Temp Probe... 178 10.14.7 Check Baby ... 179 10.14.8 Check Baby - Heat Off... 179 10.14.9 Confirm Probe Jack Connection... 180 10.14.10 Temp. Probe Failure... 180 10.14.11 System Failure ... 181 10.14.12 Weight on scales above maximum... 181 10.14.13 SpO2 alarms ... 182 10.14.13.1 Low Pulse Rate ... 182 10.14.13.2 High Pulse Rate ... 182 10.14.13.3 Low SpO2... 183 10.14.13.4 High SpO2... 183 10.14.13.5 Oximetry System Failure... 183 10.14.13.6 Check SpO2... 184 10.14.13.7 No SpO2 Probe ... 184 10.14.13.8 SpO2 Probe Off Baby ... 184 10.14.13.9 Unrecognised SpO2 Probe ... 184 10.14.13.10 Masimo SpO2 oximetry alerts... 185 10.14.14 Supply pressure alarm (blender option only)... 186 10.14.15 Alarm hierarchy... 186
Chapter 11 Preventive maintenance... 189 11.1 Repair policy ... 189 11.2 Maintenance schedule... 189 11.2.1 User maintenance... 190 11.2.2 Service maintenance ... 190 11.3 Functional testers and patient simulators... 191 11.4 Cleaning and disinfection ... 192 11.4.1 Point-of-use cleaning (cleaning during clinical use)... 193 8/232
Panda Warmer Family
M1135311 Revision K
User Manual
Contents
11.4.2 Cleaning and disinfection solutions ... 193 11.4.3 Cleaning and disinfecting the device ... 196 11.4.3.1 Cleaning and disinfection overview... 196 11.4.3.2 Preparing to clean and disinfect the device... 197 11.4.3.3 Cleaning ... 197 11.4.3.4 Disinfecting... 200 11.4.3.5 Cleaning and disinfecting accessories ... 204 11.4.3.6 Cleaning and disinfecting the disassembled bedside panel parts... 206 11.5 Dovetail rail suction tubing disassembly ... 208 11.6 Testing the supply pressure alarm... 208
Chapter 12 Specifications... 209 12.1 Mechanical specifications... 209 12.2 Electrical specifications ... 210 12.3 Power requirements and accessory outlets... 210 12.4 Operating environment ... 210 12.5 Storage conditions... 211 12.6 Shipping conditions ... 211 12.7 User control settings... 211 12.8 Irradiance ... 211 12.9 Options ... 211 12.9.1 Uninterruptible Power Supply (UPS) specifications ... 211 12.9.2 Nellcor SpO2 specifications ... 212 12.9.3 Masimo SpO2 specifications ... 212 12.9.4 T-piece resuscitation specifications... 213 12.9.5 Bag and Mask resuscitation specifications ... 215 12.9.6 Accessories (bedded only)... 216 12.10 Alarm system logs... 216
Chapter 13 Product performance... 217 13.1 System performance ... 217 13.2 Options ... 217 13.2.1 Weight scale performance (bedded only)... 217
Appendix A Legacy alarms ... 219 A.1 Silencing alarms ... 219 A.2 Baby Hot – Check Temp Probe ... 220 A.3 Baby Cold - Check Temp Probe ... 221 A.4 Check Baby... 221 A.5 Check Baby - Heat Off... 222 A.6 Confirm Probe Jack Connection ... 222 A.7 Temp. Probe Failure... 222
M1135311 Revision K
Panda Warmer Family
9/232
Contents
User Manual
A.8 System Failure ... 223 A.9 Weight on scales above maximum... 223 A.10 Alarm table ... 223
Appendix B Bedside panels ... 225 Appendix C Cleaning and disinfection... 229
10/232
Panda Warmer Family
M1135311 Revision K
1 Chapter 1 Standards and regulations 1.1 Standards The device is designed to meet the requirements of the latest recognised version of the standards that follow: • IEC 60601-1 • ANSI/AAMI ES60601-1 • CAN/CAS-C22.2 No. 60601-1 • IEC 60601-1-2 • IEC 60601-2-21 • IEC 60601-2-49 • 21 CFR CH-1 Section 1020.30 (n) Devices with the resuscitation option comply with the standards that follow, too: • ISO 10651-5 – Gas-powered emergency resuscitators • ISO 11195 – Gas mixer for medical use – standalone gas mixers Devices with the SpO2 option comply with the standards that follow, too: • ISO 80601-2-61 with the exclusion to clause 201.15.3.5.101.1 with regard to shock and vibration Devices with the ResusView™ Heart Rate option comply with the standards that follow, too: • IEC 60601-2-27 Devices with the scale option comply with the standards that follow, too: • BS EN 45501 After the installation of a replacement part, accessory or option, this device must be evaluated for compliance to these standards.
1.2 Disposal At the end of its service life, the product described in this manual, as well as its accessories, must be disposed of in compliance with the facility guidelines regulating the disposal of such products. If you have questions concerning disposal of the product, contact GE Healthcare or its representatives.
1.2.1 Patient applied parts Certain patient applied parts are intended for single use and should be disposed of properly as medical waste in accordance with regional body controlled guideline. Other patient applied parts should be cleaned according to instructions. Inspect reusable applied parts for wear, replace as necessary and dispose of used product as medical waste in accordance with regional body-controlled guidelines.
M1135311 Revision K
Panda Warmer Family
11/232
1.3 Electromagnetic compatibility (EMC) guidance
User Manual
1.3 Electromagnetic compatibility (EMC) guidance WARNING RADIO FREQUENCY INTERFERENCE HAZARD Portable and mobile RF communication equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of this equipment including cables specified by the manufacturer. Otherwise, degradation of the performance of this equipment could result. WARNING PATIENT INJURY HAZARD - INTERFERENCE Use of this equipment adjacent to or stacked with other equipment should be avoided because it could result in improper operation. If such use is necessary, this equipment and the other equipment should be observed to verify that they are operating normally. Accessories and components identified by GE Healthcare as compatible for use with this device have been tested to confirm normal operation.
1.3.1 Electromagnetic compatibility essential performance The following device performance features are considered essential to performance: • Patient skin temperature measurement accuracy • Alarms for patient measurement parameters • Patient SpO2 (oxygen saturation) and pulse rate measurement accuracy • Retention of patient data and restoration of system settings during 30 seconds or less power interruptions • Patient heart rate measurement accuracy • Display information for patient parameters (SpO2 indication of greater than 30 seconds display update time and signal inadequacy and ECG- Gain, amplitude indication and Waveform sweep speed) • Restoration of previous operating mode within 15 seconds of exposure to defibrillation energy with exception of the ResusView™ Heart Rate option • Immunity to High Frequency surgical devices for patient skin temperature and SpO2 • Patient weight measurement accuracy (if the scale is installed)
1.3.2 Degraded performance characteristics High level electromagnetic disturbance can cause degraded performances including display flicker, system restart, loss of thermoregulation, erratic or errors in parametric measurement, erroneous system failures and false alarms.
12/232
Panda Warmer Family
M1135311 Revision K
User Manual
1.3 Electromagnetic compatibility (EMC) guidance
1.3.3 Guidance and manufacturer’s declaration – electromagnetic emissions The system is intended for use in the environment specified as follows. Table 1-1 Electromagnetic emissions Emissions test RF emissions CISPR 11 RF emissions
Compliance
Electromagnetic environment guidance
Group 1
The system uses RF energy only for its internal function. Thus, its RF emissions are very low and are not likely to cause interference in nearby electronic equipment.
Class A
CISPR 11 Harmonic emissions
The system is suitable for use in all establishments, other than domestic and those directly connected to the public low voltage power supply network that supplies buildings used for domestic purposes.
Class A
IEC 61000-3-2 Voltage fluctuations/flicker emissions IEC 61000-3-3
Complies
The emissions characteristics of this equipment make it suitable for use in industrial areas and hospitals (CISPR 11 class A). If it is used in a residential environment (for which CISPR 11 class B is normally required), this equipment might not offer adequate protection to radio-frequency communication services. The user might need to take mitigation measures, such as relocating or reorienting the equipment.
1.3.4 Manufacturer’s guidance and declaration regarding electromagnetic immunity The device is intended for use in the environment specified as follows. Table 1-2 Electromagnetic immunity Immunity test
IEC 60601-1-2 test level
Compliance level
±8 kV contact
±8 kV contact
±2 kV, ±4 kV, ±8 kV, ±15 kV air
±2 kV, ±4 kV, ±8 kV, ±15 kV air
±2 kV
±2 kV
100 kHz repetition frequency
100 kHz repetition frequency
Differential Mode
Differential Mode
Surge
±0.5 kV, ±1 kV
±0.5 kV, ±1 kV
IEC 61000-4-5
Common Mode
Common Mode
±0.5 kV, ±1 kV, ±2 kV
±0.5 kV, ±1 kV, ±2 kV
Electrostatic discharge IEC 61000-4-2 Electrical fast transient/ burst IEC 61000-4-4
M1135311 Revision K
Panda Warmer Family
Electromagnetic environment guidance Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%. Mains power quality should be that of a typical commercial or hospital environment. Mains power quality should be that of a typical commercial or hospital environment.
13/232
1.3 Electromagnetic compatibility (EMC) guidance
User Manual
Table 1-2 Electromagnetic immunity (Table continued) Immunity test
Voltage dips IEC 61000-4-11
Voltage interruptions IEC 61000-4-11
IEC 60601-1-2 test level
Compliance level
Electromagnetic environment guidance
0% UT: 0.5 cycle
0% UT: 0.5 cycle
At 0", 45°, 90°, 135°, 180°, 225°, 270° and 315°
At 0", 45°, 90°, 135°, 180°, 225°, 270° and 315°
0% UT: 1 cycle and 70% UT; 25/30 cycles
0% UT: 1 cycle and 70% UT; 25/30 cycles
Single phase: at 0°
Single phase: at 0°
0% UT; 250/300 cycles
0% UT; 250/300 cycles
Mains power quality should be that of a typical commercial or hospital environment. If the user of the device requires continued operation during power mains interruptions, it is recommended that the device be powered from an uninterruptible power supply.
Power frequency (50/60 Hz) 30 A/m magnetic field environment 50 Hz or 60 Hz IEC 61000-4-8
30 A/m
Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment.
NOTE UT is the main voltage before application of the test level.
1.3.5 International Electronic Commission (IEC) guidance and manufacturer’s declaration regarding electronic immunity The warmer is intended for use in the environment specified as follows.
14/232
Panda Warmer Family
M1135311 Revision K
User Manual
1.3 Electromagnetic compatibility (EMC) guidance
Table 1-3 IEC electromagnetic immunity Immunity test
IEC 60601-1-2 test level
Compliance level
Electromagnetic environment guidance
Conducted RF
3 Vrms
3 Vrms
IEC 61000-4-6
6 Vrms in ISM bands between 0.15 MHz and 80 MHz
6 Vrms
Portable and mobile RF communications equipment should be used no closer to any part of the warmer, including cables, than the recommended separation distance calculated from the equation applicable for the frequency of the transmitter.
80% AM at 1 kHz 3 V/m
Radiated RF
3 V/m
IEC 61000-4-3
80 MHz to 2.7 GHz
d=1.2√P
80% AM to 1 kHz
Radiated RF can affect the accuracy of in-bed scale readings. However, the in-bed scale is not critical to the performance of the warmer unit.*1
Compliance to Table 9 of IEC 60601-1-2:2014/EN 60601-1-2:2015 IEC 60601-2-21
Recommended separation distance:
d=1.2√P = 26 MHz to 800 MHz
3 V/m
3 V/m
d=2.3√P = 800 MHz to 2.7 GHz
26 MHz to 1 GHz
normal operation
10 V/m
10 V/m
26 MHz to 1 GHz
no hazard
where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in metres (m). Field strengths for fixed RF transmitters as determined by an electromagnetic site survey*2 should be less than the compliance level in each frequency range.*3 Interference may occur in the vicinity of equipment marked with the following symbol:
*1 Portable and mobile equipment can affect medical electronic equipment. *2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. Field strengths from fixed transmitters such as base stations for radio, cellular/cordless telephones and land mobile radios, amateur radios, AM and FM radio broadcasts and TV broadcasts cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the warmer unit is used exceeds the applicable RF compliance level above, the unit should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the warmer. *3 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. Over the frequency range 150 KHz to 80 MHz, field strengths should be less than 3 V/m.
NOTE At 80 MHz and 800 MHz, the higher frequency applies.
M1135311 Revision K
Panda Warmer Family
15/232
1.4 Vermont RoHS
User Manual
1.3.6 Recommended separation distance between portable and mobile RF communications equipment and the system The system is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The user can prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the system as recommended as follows, according to the maximum output power of the communications equipment. Table 1-4 Separation distance Separation distance in metres (m) according to frequency of transmitter
Rated maximum output power of transmitter (W)
150 kHz to 80 MHZ d = 1.2 P
80 MHz to 800 MHz d = 1.2 P
800 MHz to 2.7 GHz d = 2.3 P
0.01
0.12
0.12
0.23
0.1
0.38
0.38
0.73
1.0
1.2
1.2
2.3
10.0
3.8
3.8
7.3
100.0
12.0
12.0
23.0
For transmitters rated at a maximum output power not listed above, the recommended separation distance (d) in metres (m) can be determined using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and persons.
1.4 Vermont RoHS The fluorescent backlight display (2094207-001) on legacy models (manufactured before 2017) of this device contains mercury and must be recycled or discarded in compliance with local, state or country laws. This is not applicable to versions of the warmer manufactured in or after 2017. This is also not applicable to warmers that have the LED display upgrade kit installed. Refer to the Panda Warmer Family and Giraffe Warmer Service Manual to identify whether the upgrade kit has been installed.
1.5 CE mark The first year that the device had the CE mark was 2018.
16/232
Panda Warmer Family
M1135311 Revision K
2 Chapter 2 Introduction 2.1 Manual availability More copies of this manual are available on request from the GE Healthcare office that is included on the rear cover of this manual.
2.2 Intended users The intended users of this manual are the end users of the equipment, primarily caregivers in general infant care areas (such as Labour & Delivery and NICU), hospital biomedical engineers and hospital cleaning staff.
2.3 User responsibility The device operates as this manual and related labels or inserts describe when the supplied instructions to assemble, operate, do the maintenance and repair the device are obeyed. You must periodically check the device. Do not use a defective device. Immediately replace all parts that are broken, missing, worn, damaged or have contamination. If such repair or replacement becomes necessary, call or write to the nearest GE Healthcare Regional Service Centre for service recommendations. Only GE Healthcare qualified personnel must repair the device and its parts according to the written instructions supplied by GE Healthcare. The user of the device is responsible for all malfunctions that occur because of incorrect use, incorrect maintenance, incorrect repair, damage or change by a person other than GE Healthcare.
2.4 Abbreviations and acronyms Table 2-1 Abbreviation or acronym Meaning bpm
beats per minute
CF
cardiac floating
cm-H2O
a unit of pressure measured in centimetres of water
ECG
electrocardiogram
ESD
electrostatic discharge electrostatic sensitive device
FiO2
fraction of inspired oxygen
g
grams
HFOV
high frequency oscillating ventilation
HR
heart rate
IFU
instructions for use
IV
intravenous
L/min
litres per minute
M1135311 Revision K
Panda Warmer Family
17/232
2.5 Terminology
User Manual
Table 2-1 (Table continued) Abbreviation or acronym Meaning LED
light-emitting diode
mm-Hg
a unit of pressure measured in millimetres of mercury
NICU
neonatal intensive care unit
PEEP
positive end expiratory pressure
pH
power of Hydrogen
PIP
peak inspiratory pressure
PPE
personal protection equipment
RoHS
Restriction of Hazardous Substances
SpO2
blood oxygen saturation level
UPS
uninterruptible power supply
2.5 Terminology This section identifies terms that may be used for some devices in the system. Single Patient Use Devices The single patient use device is not designed or validated to be reused. Reuse may cause a risk of cross-contamination, affect the measurement accuracy, system performance or cause a malfunction as a result of the device being physically damaged due to cleaning, disinfection, resterilisation or reuse. A reuse of this device on the same patient, with periods of use separated by a period of non-use, is acceptable. The user must ensure that the device is not damaged or contaminated between usages on the same patient. Single-Use Devices The single use device is used on 1 patient during only 1 procedure and then discarded. Reuse may cause a risk of cross-contamination, affect the measurement accuracy, system performance or cause a malfunction as a result of the device being physically damaged due to cleaning, disinfection, re-sterilisation or reuse.
2.6 Illustrations All illustrations are provided as examples only. Your device may not be equipped with the specific feature shown. In addition, unless explicitly stated, the display examples may not represent your equipment setup or displayed data.
18/232
Panda Warmer Family
M1135311 Revision K
3 Chapter 3 Safety 3.1 Safety definitions The following definitions are used to classify precautions on product safety in this manual. Failure to observe precautions could result in injury to people or damage to property. DANGER Indicates a hazardous situation that, if not avoided, will result in death WARNING Indicates a hazardous situation that, if not avoided, could result in death or serious injury CAUTION Indicates a potential hazard that, if not avoided, could result in minor injury NOTICE Indicates a potential situation that, if not avoided, could result in equipment damage NOTE Notes are used to draw attention to important information.
3.2 Safety signs Mandatory action signs These signs indicate a mandatory action and are identified by a blue background and white symbol. Table 3-1 Mandatory action signs Description
Symbol standard reference
General mandatory action sign
ISO 7010-M001
Prohibition signs These signs indicate prohibited actions and are identified by a red cross-out circle.
M1135311 Revision K
Panda Warmer Family
19/232
3.3 Symbols displayed on the device
User Manual
Table 3-2 Prohibition signs Description
Symbol standard reference
General prohibition sign
ISO 7010-P001
Warning signs These signs are identified by a yellow background, black triangular band and a black symbol. Table 3-3 Warning signs Description
Symbol standard reference
General warning sign
ISO 7010-W001
3.3 Symbols displayed on the device The following symbols may be used on the device, the device labels (including documentation) or the device packaging. Not all symbols may be used with a specific device. Table 3-4 Symbols displayed on the device Symbol
Usage
Symbol standard reference
Active alarm indicator (yellow or red)
-
Air tank pressure
-
Airway pressure manometer
-
Alarm off
IEC 60417-5319 Definition deviated by IEC 60601-1-8
20/232
Panda Warmer Family
M1135311 Revision K
User Manual
3.3 Symbols displayed on the device
Table 3-4 Symbols displayed on the device (Table continued) Usage
Symbol standard reference
Alternating current
IEC 60417-5032
Audio alarm
IEC 60417-5013
Audio alarm paused – temporary
IEC 60417-5576-2
Authorised Representative in the European community
ISO 15223-1
Baby
IEC 60417-5667
Bag and mask
-
LOT
Batch code
ISO 7000-2492
REF
Catalogue number
ISO 7000-2493
Clockwise rotation increases magnitude
–
Consult instructions for use
ISO 7000-1641
Control down
-
Control up
-
CSA Group certification mark to US and Canadian standard
-
Date of manufacture
ISO 7000-2497
Symbol
EC
REP
® C
M1135311 Revision K
US
Panda Warmer Family
21/232