Basic User Manual
662 Pages
Preview
Page 1
GE Healthcare
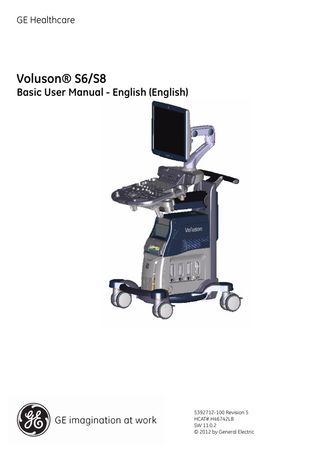
Voluson® S6/S8 Basic User Manual - English (English)
5392712-100 Revision 5 HCAT# H46742LB SW 11.0.2 © 2012 by General Electric
Revision History
i-ii
Revision
Date
Revision 1
October 2010
Revision 2
December 2010
Revision 3
May 2011
Revision 4
September 2011
Revision 5
August 2012
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
Chapter 1 - General General Contacting GE Healthcare Ultrasound - - - - - - - - - - - - - - - - - - - - - - - - 1-3 Manufacturer - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-5 About this User Manual - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-5
Chapter 2 - Safety Safety Classification - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-5 Remarks for Safe Use - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-6 System Safety and Maintenance - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-7 Biopsy Safety and Maintenance - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-19 Manufacturer Responsibility- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-20 Service Documents- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-21 Bioeffects and Safety of Ultrasound Scans - - - - - - - - - - - - - - - - - - - - 2-21 Disposal- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-24
Chapter 3 - Description of the System Description of the System Product Description- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-2 System Assembly - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-3 Mechanical Adjustment - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-4 Layout of Menus - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-7 Remove USB Devices- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-18 Electronic User Manual (EUM) - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-19
Chapter 4 - Operating the System Operating the System General Remarks - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-2 Safety Warnings - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-2 Power Off / Shutdown - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-3 Transducer Connection - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-5 Prepareing the Transducer - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-6
Chapter 5 - Probes and Biopsies Probes and Biopsies Probes- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-2 Biopsies- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-8
Chapter 6 - 2D Mode 2D Mode B-Flow - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-24
Chapter 7 - Motion Mode M Mode M Main Menu - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7-2 M Operation - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7-3 M Sub Menu- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7-6 Anatomical M-Mode (AMM) - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7-23
Chapter 8 - Doppler Modes Doppler Modes Pulsed Wave Doppler Mode (PW Mode) - - - - - - - - - - - - - - - - - - - - - - - 8-2 Color Flow Mode (CFM) - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-8
Chapter 9 - Volume Mode Volume Mode Volume Acquisition with Volume Probes - - - - - - - - - - - - - - - - - - - - - - - 9-3 Volume Acquisition: Static 3D Sectional Planes - - - - - - - - - - - - - - - - - 9-17
Chapter 10 - Elastography Mode Elastography Mode GUI elements - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 10-2 Elastography Main Menu - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 10-4
Chapter 11 - Measurements and Patient Worksheets
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
i-iii
Measurements and Patient Worksheets (Reports) Generic Measurements - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 11-2 Measure Setup - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 11-99
Chapter 12 - Archive Archive Current Patient Dialog - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 12-3 Exam Review - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 12-33 Selecting Exams- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 12-41 Settings - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 12-42
Chapter 13 - Utilities and System Setup Utilities and System Setup Utilities- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 13-2
Chapter 14 - Programmable Keys Programmable Keys Where to program the keys - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 14-2 Start Exam Button- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 14-9 End Exam Button - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 14-10
Chapter 15 - Connections Connections How to Connect Auxiliary Devices Safely - - - - - - - - - - - - - - - - - - - - - 15-2 To Connect Internal and External Accessories- - - - - - - - - - - - - - - - - - 15-3 Connection between Internal I/O and External I/O - - - - - - - - - - - - - - - 15-4 Connection of Peripherals - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 15-8 External 19’’ Monitor - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 15-9 Isolating transformer Noratel IMED 300WR - - - - - - - - - - - - - - - - - - 15-10
Chapter 16 - Technical Data Technical Data / Information Safety Conformance - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 16-2 Physical Attributes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 16-4 System overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 16-5 Display Modes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 16-6 Display Annotation - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 16-7 System Standard Features - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 16-7 System Options - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 16-9 System Parameters - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 16-10 Scanning Parameters - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 16-14 Generic Measurements and Measurements/Calculations - - - - - - - - - 16-21
Chapter 17 - ANNEX ANNEX- Abbrevations
i-iv
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
Chapter 1 General This chapter consists of information concerning indications for use and contact information.
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
1-1
General
1. General
The Voluson® S6/S8 is a professional diagnostic Ultrasound System which transmits Ultrasound waves into body tissues and forms images from the information contained within the received echoes. The Voluson® S6/S8 is an Active Diagnostic Medical Product belonging to Class IIa according to the MDD 93/42/EWG regulation for use on human patients. The Voluson® S6/S8 is developed and produced by GE Healthcare. For more Information, please contact: GE Healthcare Telephone
+(82) 31-740-6273
Internet
http://www.gehealthcare.com
65-1, Sangdaewon-dong, Jungwon-gu, Seongnam-si, Gyeonggi-do, 462-120, Korea Authorized EU Representative GE Medical Systems Information Technologies GmbH Munzingerstrasse-5, 79111 Freiburg, Germany Dear Valuable Customer, We herewith would like to inform you that the American Institute of Ultrasound in Medicine (AIUM) advocates the responsible use of diagnostic ultrasound. The AIUM strongly discourages the non-medical use of ultrasound for psychosocial or entertainment purposes. The use of either two-dimensional (2D) or three-dimensional (3D) ultrasound to only view the fetus, obtain a picture of the fetus or determine the fetal gender without a medical indication is inappropriate and contrary to responsible medical practice. Although the general use of ultrasound for medical diagnosis is considered safe, ultrasound energy has the potential to produce biological effects. Ultrasound bioeffects may result from scanning for a prolonged period, inappropriate use of color or pulsed Doppler ultrasound without a medical indication, or excessive thermal or mechanical index settings (American Institute of Ultrasound in Medicine: Keepsake Fetal Imaging; 2005). Thus ultrasound should be used in a prudent manner to provide medical benefit to the patient.
1-2
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
General 1.1 Contacting GE Healthcare Ultrasound For additional information or assistance, please contact your local distributor or the appropriate support resource listed on the following pages.
INTERNET
http://www.gehealthcare.com http://www.gehealthcare.com/usen/ultrasound/products/ probe_care.html
USA
GE Healthcare TEL: (1) 800-437-1171 Ultrasound Service Engineering FAX: (1) 414-721-3865 9900 Innovation Drive Wauwatosa, WI 53226
Clinical Questions
For information in the United States, Canada, Mexico and parts of the Caribbean, call the Customer Answer Center TEL: (1) 800-6825327 or (1) 262-524-5698 In other locations, contact your local Applications, Sales or Service Representative.
Service Questions
For service in the United States, call GE CARES TEL: (1) 800-4371171 For service for compact products in the United States, call TEL: (1) 877-800-6776 In other locations, contact your local Service Representative.
Accessories Catalog Requests
To request the latest GE Accessories catalog or equipment brochures in the United States, call the Response Center TEL: (1) 800-643-6439 In other locations, contact your local Applications, Sales or Service Representative.
Placing an Order
To place an order, order supplies or ask an accesory-related question in the United States, call the GE Access Center TEL: (1) 800-472-3666 In other locations, contact your local Applications, Sales or Service Representative.
CANADA
GE Healthcare TEL: (1) 800-664-0732 Ultrasound Service Engineering 9900 Innovation Drive Wauwatosa, WI 53226 Customer Answer Center TEL: (1) 262-524-5698
EUROPE
GE Ultraschall TEL: 0130 81 6370 toll free Deutschland GmbH & Co. KG TEL: (33) 130.831.300 Beethovenstrasse 239 FAX: (49) 212.28.02.431 Postfach 11 05 60
ASIA
GE Ultrasound Asia (Singapore) TEL: 65-291 8528 Service Department - Ultrasound FAX: 65-272-3997 298 Tiong Bahru Road #15-01/06 Central Plaza Singapore 169730
ARGENTINA
GEME S.A. TEL: (1) 639-1619 Miranda 5237 FAX: (1) 567-2678 Buenos Aires - 1407
AUSTRIA
GE GesmbH Medical Systems Austria TEL: 0660 8459 toll free Prinz Eugen Strasse 8/8 FAX: +43 1 505 38 74 A-1040 WIEN TLX: 136314
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
1-3
General
1-4
BELGIUM
GE Medical Systems Benelux TEL: 0 800 11733 toll free Gulkenrodestraat 3 FAX: +32 0 3 320 12 59 B-2160 WOMMELGEM TLX: 72722
BRAZIL
GE Sistemas Medicos TEL: 0800-122345 Av Nove de Julho 5229 FAX: (011) 3067-8298 01407-907 Sao Paulo SP
CHINA
GE Healthcare - Asia TEL: (8610) 5806 9403 No. 1, Yongchang North Road FAX: (8610) 6787 1162 Beijing Economic & Technology Development Area Beijing 100176, China
DENMARK
GE Medical Systems TEL: +45 4348 5400 Fabriksparken 20 FAX: +45 4348 5399 DK-2600 GLOSTRUP
FRANCE
GE Medical Systems TEL: 05 49 33 71 toll free 738 rue Yves Carmen FAX: +33 1 46 10 01 20 F-92658 BOULOGNE CEDEX
GERMANY
GE Ultraschall TEL: 0130 81 6370 toll free Deutschland GmbH & Co. KG TEL: (49) 212.28.02.207 Beethovenstrasse 239 FAX: (49) 212.28.02.431 Postfach 11 05 60 D-42655 Solingen
GREECE
GE Medical Systems Hellas TEL: +30 1 93 24 582 41, Nikolaou Plastira Street FAX: +30 1 93 58 414 G-171 21 NEA SMYRNI
ITALY
GE Medical Systems Italia TEL: 1678 744 73 toll free Via Monte Albenza 9 FAX: +39 39 73 37 86 I-20052 MONZA TLX: 3333 28
LUXEMBOURG
TEL: 0800 2603 toll free
MEXICO
GE Sistemas Medicos de Mexico S.A. de C.V. Rio Lerma #302, 1° y 2° Pisos TEL: (5) 228-9600 Colonia Cuauhtemoc FAX: (5) 211-4631 06500-Mexico, D.F.
NETHERLANDS
GE Medical Systems Nederland B.V. TEL: 06 022 3797 toll free Atoomweg 512 FAX: +31 304 11702 NL-3542 AB UTRECHT
POLAND
GE Medical Systems Polska TEL: +48 2 625 59 62 Krzywickiego 34 FAX: +48 2 615 59 66 P-02-078 WARSZAWA
PORTUGAL
GE Medical Systems Portuguesa S.A. TEL: 05 05 33 7313 toll free Rua Sa da Bandeira, 585 FAX: +351 2 2084494 Apartado 4094 TLX: 22804 P-4002 PORTO CODEX
RUSSIA
GE VNIIEM TEL: +7 095 956 7037 Mantulinskaya UI. 5A FAX: +7 502 220 32 59 123100 MOSCOW TLX: 613020 GEMED SU
SPAIN
GE Healthcare TEL: +34 (91) 663 25 00 Avda. Europa, 22 FAX: +34 (91) 663 25 01 E-28108 Alcobendas, Madrid
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
General SWEDEN
GE Medical Systems TEL: 020 795 433 toll fre PO-BOX 1243 FAX: +46 87 51 30 90 S-16428 KISTA TLX: 12228 CGRSWES
SWITZERLAND
GE Medical Systems (Schweiz) AG TEL: 155 5306 toll fre Sternmattweg 1 FAX: +41 41 421859 CH-6010 KRIENS
TURKEY
GE Medical Systems Turkiye A.S. TEL: +90 212 75 5552 Mevluk Pehliran Sodak FAX: +90 212 211 2571 Yilmaz Han, No 24 Kat 1 Gayretteppe ISTANBUL
UNITED KINGDOM
GE Medical Systems TEL: 0800 89 7905 toll free Coolidge House FAX: +44 753 696067 352 Buckingham Avenue SLOUGH Berkshire SL1 4ER
OTHER COUNTRIES
NO TOLL FREE TEL: international code + 33 1 39 20 0007
1.2 Manufacturer GE Ultrasound Korea, Ltd. 65-1, Sangdaewon-dong, Jungwon-gu, Seongnam-si, Gyeonggi-do, 462-120 KOREA
1.3 About this User Manual •
Read and understand all instructions in the Basic User Manual before attempting to use the Voluson® S6/S8.
•
This Manual has to be used in connection with the Voluson® S6/S8.
•
Keep this User Manual with the equipment at all times.
•
All information contained in the Voluson® S6/S8 User Manual is relevant.
•
Periodically review the procedures for operation and safety precautions.
Please note that orders are based on the individually agreed specifications and may not contain all features listed in this manual.
The screen graphics and illustrations in this manual are for illustrational purposes only and may be different from what you see on the screen or device
All references to standards / regulations and their revisions are valid for the time of publication of the user manual.
It might be possible that some probes, options or features are NOT available in some countries.
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
1-5
General This Page was Intentionally Left Blank.
1-6
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
Chapter 2 Safety Describes the safety and regulatory information pertinent for operating this ultrasound system.
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
2-1
Safety
2. Safety
The Voluson® S6/S8 scanner system has been designed for utmost safety for patient and user. Read the following chapters thoroughly before you start working with the machine! The manufacturer guarantees safety and reliability of the system only when all the following cautions and warnings are observed. INDICATIONS FOR USE This system is intended for use by a qualified physician for ultrasound evaluation in the following clinical applications: Image Acquisition for diagnostic purposes incl. measurements on acquired image. Clinical applications:
Patient population:
Operator profile:
• Fetal/Obstetrics • Abdominal/GYN (including infertility monitoring of follicle development) • Pediatric • Small Organ (breast, testes, thyroid, etc.) • Cardiac (fetal cardio) • Peripheral Vascular • Musculo-skeletal Conventional and Superficial • Transvaginal and Transrectal
• Age: all ages (encl. embryos and fetuses) • Location: worldwide • Sex: male and female • Weight: all weight categories
• Qualified and trained physicians or sonographers with at least basic ultrasound knowledge. • The operator must have read and understood the user manual.
CONTRAINDICATIONS The Voluson® S6/S8 system is not intended for: •
ophthalmic use or any use causing the acoustic beam to pass through the eye.
•
The system is not intended for intra-operative use except vagina and rectum.
Federal law restricts this device to sale by or on the order of a physician!
2-2
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
Safety 2.1 Warning labels used in the Basic User Manual Describes general precautions necessary to protect health and the equipment.
Describes important information that has to be read before proceeding.
Describes precautions necessary to prevent the risk of disease transmission or infections.
Describes precautions necessary to prevent the risk of injury through electric hazards.
Describes precautions necessary to prevent the risk of injury through explosion hazard!
Describes precautions necessary to prevent the risk of injury through moving or tipping hazard!
Describes precautions necessary to prevent the risk of injury through mechanical hazard!
2.2 Symbols Used Some symbols used with electrical medical equipment have been accepted as standard by IEC. They serve for marking connections, accessories, and as warnings.
Stand-by
Insulated patient application part (Type BF)
Mains power switch ON
Mains power switch OFF
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
2-3
Safety Do not use the following devices near this equipment: cellular phone, radio receiver, mobile radio transmitter, radio controlled toy, broadband power lines,etc. Use of these devices near this equipment could cause this equipment to perform outside the published specifications. Keep power to these devices turned off when near this equipment.
Protective earth (ground) connection
Potential equilibrium connection
UL conformity mark according to UL 60601-1 and CAN/CSA C22/2 NO. 601.1:
Protection against the effects of immersion
Caution, consult accompanying documents. This symbol advises the reader to consult the accompanying documents for important safety related information such as warnings and pre-cautions that cannot be presented on the device itself.
Dangerous electric voltage. Unplug the main plug before opening the system!
Disposal: See ‘Disposal’ on page 2-24 for more information.
CE Conformity mark according to Medical Device Directive 93/42/EEC
This product consists of devices that may contain mercury, which must be recycled or disposed of in accordance with local, state, or country laws. (Within this system, the backlight lamps in the monitor display, contain mercury.)
This symbol signifies that the user manual must be read
Tipping danger. Do not lean on the cart and take special care when movingl
DO NOT place a finger, hand or any object on the joint of the monitor or monitor arm to avoid injury when moving the monitor and monitor arm.
The monitor has to be secured with the monitor-transportlock when moving or transporting.
2-4
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
Safety
These symbols indicate that at least one of the six hazardous substances of the China RoHS Labelling Standard is above the RoHS limitation. The number inside the circle is referred to as the Environmental Friendly Use Period (EFUP). It indicates the number of years that the product, under normal use, will remain harmless to health of humans or the environment. EFUP = 10 for Short Use Products EFUP = 20 for Medium Use Products
This symbol is attached on the rear part of the system to indicate required caution and information. The machine should be used in compliance with law some jurisdictions restrict certain use such as gender determination.
This label is printed on the packing box of the system to indicate the humidity, temperature and air pressure condition for the storage and shipment.
2.3 Classification Classifications Type of protection against electric shock •
Class I Equipment (*1)
Degree of protection against electric shock •
Type BF Applied part (*2) (for all Probes)
Continuous Operation System is Ordinary Equipment (IPX0) Footswitch is IPX8 Probe head (immersible portion) is IPX7
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
2-5
Safety *1. Class I Equipment EQUIPMENT in which protection against electric shock does not rely on BASIC INSULATION only, but includes an earth ground. This additional safety precaution prevents exposed metal parts from becoming LIVE in the event of an insulation failure. *2. Type BF Applied Part TYPE BF APPLIED PART providing a specified degree of protection against electric shock, with particular regard to allowable LEAKAGE CURRENT.
2.4 Remarks for Safe Use •
Get acquainted with the transducers and the ultrasound system: read the user manual thoroughly!
•
Misinterpretation of an Ultrasound Image can lead to false diagnosis.
•
Follow all safety instructions as well as the clinically adopted precautions and measures for hygiene.
•
Any ultrasound transducers - irrespective of system and design - are sensitive to shock and shall be treated with care. Pay attention to cracks, which may allow conductive fluids to leak in.
•
Avoid kinking, bending or twisting of probe cables and take care to guard them against mechanical stress (e.g., wheels or heels).
•
The probes must not be exposed to mechanical shock (e.g., by dropping). Any damage caused in this will void the warranty!
•
Have the scanner system and the transducers regularly checked (for faulty cables, housing, etc.) by authorized personnel!
•
Damage to transducer or cable may lead to a safety hazard, therefore have them repaired immediately!
•
Before plugging in or unplugging a transducer, activate the “FREEZE” mode!
•
A specialist familiar with the handling and use of the system shall perform installation and first switch-on and check-up of the system.
•
For safety reason, avoid handling fluids in the vicinity of the system. Fluids leaking into the disk drive can damage the drive.
•
The user manual must always be with the scanner system. It is the user’s duty to ensure this!
•
Only probes conforming to type BF requirements may be used with the Voluson® S6/ S8.
•
Do not install software on the system, that has not been released by GE Healthcare, as this may lead to erroneous data transfer and thereby decrease system performance.
•
The Voluson® S6/S8 system has been tested for EMC and is compliant with EN 55011 group 1 class B (CISPR 11 amendment 1) and EN 60601-1-2. The Voluson® S6/S8 system is approved for use in a residential district. It is expected that the user has medical experience and is well informed with the user manual.
•
Main power quality should be that of a typical commercial and/or hospital environment. If the user requires continued operation during power main interruption, it is recommended that the system be powered from an uninterruptable power source (UPS). There have been reports of severe allergic reactions to medical devices containing latex (natural rubber). Operators are advised to identify latex-sensitive patients and be prepared to treat allergic reactions promptly. Refer to FDA Medical Alert MDA91-1.
2-6
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
Safety 2.5 System Safety and Maintenance This machine should be used in compliance with the law. Some jurisdictions restrict certain uses such as gender determination.
Federal law restricts this device to sale by or on the order of a physician.
2.5.1 Instructions for Use This equipment has been tested and found to comply with the limits for medical devices in IEC 60601-1-2. These limits are designed to provide reasonable protection against harmful interference in a typical medical installation. This equipment generates, uses and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to other devices in the vicinity. However, there is no guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interference to other devices, which can be determined by turning the equipment off and on, the user is encouraged to try to correct the interference by one or more of the following measures: •
Reorient or relocate the device.
•
Increase the distance between equipment.
•
Connect the equipment to an outlet on a circuit different from that to which the other device(s) are connected.
•
Consult the manufacturer or field service technician for help.
The Voluson S6/S8 does not contain any operator serviceable internal components. Ensure that unauthorized personnel do not tamper with the unit.
Do not touch the patient and the Signal Input/Output lines (for example, USB port) at the same time.
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
2-7
Safety 2.5.2 Environmental Conditions for Operation
NOTE:
Temperature:
18°C to 30°C resp. 64°F to 86°F
Humidity:
30% to 80% RH, no condensation
Barometric pressure:
700 to 1060 hPa
Light conditions:
natural & artificial light source*
Maximum operating altitude:
3000m
Pollution degree:
2
Overvoltage category:
II
Material group:
IIIB
Total audible noise emission:
<55dB
* Bright light could impact readability of screen. Ultrasound systems are highly sensitive medical instruments that can easily be damaged by improper handling. Use care when handling and protect from damage also when not in use. DO NOT use a damaged or defective ultrasound system. Failure to follow these precautions can result in serious injury and equipment damage. This equipment is not to be used during transportation (e.g. ambulance cars, aircrafts).
Thermal Safety. Maintaining a safe thermal environment for the patient has been a design priority at GE Healthcare. Software settings limit the power dissipated for the ultrasound transducer as well as the motor-drive to values low enough to ensure that operating temperatures stay below 43°C. Not to be used in sterile environment.
This equipment must not be used in oxygen enriched atmosphere or in the presence of inflammable gases (e.g. anesthetic gases).
The use of the system outside the described conditions or intended use, and disregarding safety related information is considered as abnormal use. The manufacturer is not liable for damage caused by abnormal use of the device! Use for diagnostic purposes only.
2-8
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
Safety Do not operate the system in the vicinity of a heat source, of strong electric or magnetic fields (close to a transformer), or near instruments generating high-frequency signals, such as HF surgery. These can affect the ultrasound images adversely. In the event the equipment has been brought from a cold environment (stock room, airfreight) into a warm room, allow several hours for temperature balance and passing of condensation humidity before switching on for the first time. Do not cover the ventilation holes of the Voluson® S6/S8!
2.5.2.1 Electric Installation The system must be exclusively installed in medically used rooms. The equipment conforms with regulations for electrical safety (IEC 60601) and safety class IIa according to the MDD 93/42/EWG regulation for use on humans patients. Probes are rated Type BF. Local safety regulations may require an additional connection between the potential equilibrium bolt and the building’s grounding system. Before switching on the first time, the local main voltage and frequencies have to be checked against the values indicated on the Voluson® S6/S8 rating plate located on the rear panel. Only authorized personnel must perform any change to the system. The minimum required house installation must have 16A. Do not detach power cord from Voluson S6/S8. To avoid risk of electric shock, Voluson S6/ S8 must only be connected to a supply mains with PROTECTIVE EARTH. Do not connect any unauthorized equipment between power cord plug and wall power outlet. 2.5.2.2 Moving or lifting the System Moving the system on planes
Moving the system on inclines *
The Voluson® S6/S8 weighs 90 kg or more, depending on installed peripherals, (200 lbs., or more) when ready for use. Care must be used when moving it or replacing its parts. Failure to follow the precautions listed could result in injury, uncontrolled motion and costly damage. ALWAYS: • Use the handle to move the system. • Be sure the pathway is clear. • Use slow, careful motions. • Do not let the system strike walls or door frames. * Two people are required when moving on inclines or lifting more than 16 kg (35 lbs).
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5
2-9
Safety Always place the system on horizontal ground and block the front wheels. The device might tipp over or roll away. See ‘Caster Brakes’ on page 3-6 for more information.
Lower the console to its minimal height when moving or transporting the system.
Handle carefully. A drop of more than 5 cm can cause mechanical damages.
The monitor has to be secured with the monitor-transportlock when moving or transporting the system. See ‘Mechanical Adjustment’ on page 3-4 for more information. Process cautiously when crossing door or elevator thresholds. Use the handle to push/ pull the system, e.g., do not use the LCD. Failure to do so may cause serious injury or system damage. There is a pinch point on the LCD monitor. Take care to avoid injuring hands or fingers when flipping down the LCD monitor.
2.5.3 Cleaning and Maintenance Prior to cleaning any part of the system: 1.
Turn off the system power. If possible, disconnect the power cord.
To clean the system cabinet: 1.
Moisten a soft, non-abrasive folded cloth with a mild,general purpose, non-abrasive soap and water solution.
2.
Wipe down the top, front, back, and both sides of the system cabinet.
Do not spray any liquid directly into the unit.
To clean the monitor face: Use a soft, folded cloth. Gently wipe the monitor face. Do NOT use a glass cleaner that has a hydrocarbon base (such as Benzene, Methyl Alcohol or Methyl Ethyl Ketone) on monitors with the filter (anti-glare shield). Hard rubbing will also damage the filter. When cleaning the monitor, make sure not to scratch the monitor.
To clean the operator control panel:
2-10
1.
Moisten a soft, non-abrasive folded cloth with a mild,general purpose, non-abrasive soap and water solution.
2.
Wipe down operator control panel.
Voluson® S6/S8 Basic User Manual 5392712-100 Revision 5