Integra LifeSciences Corporation
CUSA Surgical Aspirator Systems
CUSA NXT Neuro Handpieces and Tips Guide July 2015
Guide
2 Pages
Preview
Page 1
Integra®
CUSA NXT ®
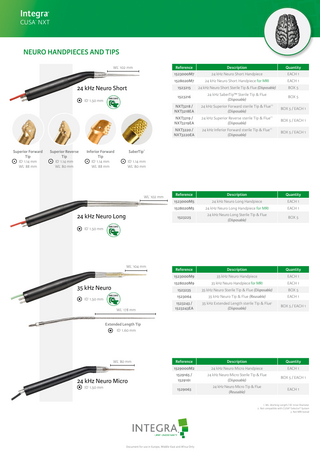
NEURO HANDPIECES AND TIPS WL 102 mm 1
24 kHz Neuro Short ID 1.50 mm 1
Superior Forward Tip ID 1.14 mm WL 88 mm 1
1
Superior Reverse Tip ID 1.14 mm WL 80 mm 1
1
MRI SAFE
Inferior Forward Tip ID 1.14 mm WL 88 mm
Quantity
1523000M7
24 kHz Neuro Short Handpiece
EACH 1
1528020M7
24 kHz Neuro Short Handpiece for MRI
EACH 1
1523215
24 kHz Neuro Short Sterile Tip & Flue (Disposable)
BOX 5
1523216
24 kHz SaberTip™ Sterile Tip & Flue (Disposable)
BOX 5
NXT3218 / NXT3218EA
24 kHz Superior Forward sterile Tip & Flue (Disposable)
NXT3219 / NXT3219EA
24 kHz Superior Reverse sterile Tip & Flue (Disposable)
NXT3220 / NXT3220EA
24 kHz Inferior Forward sterile Tip & Flue (Disposable)
Reference
Description
Quantity
1523000M5
24 kHz Neuro Long Handpiece
EACH 1
1528020M5
24 kHz Neuro Long Handpiece for MRI
EACH 1
1523225
24 kHz Neuro Long Sterile Tip & Flue (Disposable)
BOX 5
Reference
Description
Quantity
1523000M9
35 kHz Neuro Handpiece
EACH 1
1528020M9
35 kHz Neuro Handpiece for MRI
EACH 1
1523235
35 kHz Neuro Sterile Tip & Flue (Disposable)
BOX 5
1523064
35 kHz Neuro Tip & Flue (Reusable)
EACH 1
1523243 / 1523243EA
35 kHz Extended Length sterile Tip & Flue (Disposable)
BOX 5 / EACH 1
Reference
Description
Quantity
1529000M2
24 kHz Neuro Micro Handpiece
EACH 1
1529165 / 1529161
24 kHz Neuro Micro Sterile Tip & Flue (Disposable)
BOX 5 / EACH 1
1529063
24 kHz Neuro Micro Tip & Flue (Reusable)
EACH 1
2/3
BOX 5 / EACH 1
2/3
BOX 5 / EACH 1
2/3
BOX 5 / EACH 1
™
ID 1.14 mm WL 80 mm 1
1
1
WL 102 mm 1
24 kHz Neuro Long 1
Description
SaberTip
1
ID 1.50 mm
Reference
MRI SAFE
WL 104 mm 1
35 kHz Neuro ID 1.50 mm
MRI SAFE
1
WL 178 mm 1
3
Extended Length Tip ID 1.60 mm 1
WL 80 mm 1
24 kHz Neuro Micro ID 1.50 mm 1
1. WL: Working Length / ID: Inner Diameter 2. Not compatible with CUSA® Selector® System 3. Not MRI tested
Document for use in Europe, Middle-East and Africa Only
Integra®
CUSA NXT ®
LIVER HANDPIECES AND TIPS WL 94 mm 1
35 kHz Straight ID 2 mm 1
WL 146 mm
Reference
Description
Quantity
1523000M3
35 kHz Straight Handpiece
EACH 1
1523014
35 kHz Straight Standard Tip (Reusable)
EACH 1
1523043
35 kHz Straight Flues (Disposable)
BOX 20
Reference
Description
Quantity
1523000M1
24 kHz Straight Handpiece
EACH 1
1523013
24 kHz Straight Standard Tip (Reusable)
EACH 1
1523042
24 kHz Straight Flues (Disposable)
BOX 20
Reference
Description
Quantity
1523000M2
24 kHz Angled Handpiece
EACH 1
1523015
24 kHz Angled Standard Tip (Reusable)
EACH 1
1523042
24 kHz Angled Flues (Disposable)
BOX 20
Reference
Description
Quantity
1523000M6
24 kHz Laparoscopic Handpiece
EACH 1
1523029
24 kHz Laparoscopic Tip (Reusable)
EACH 1
1523032
24 kHz Laparoscopic Flues (Disposable)
BOX 10
1
24 kHz Straight ID 2 mm 1
WL 141 mm 1
24 kHz Angled ID 2 mm 1
WL 115 mm 1
24 kHz Laparoscopic ID 2 mm 1
Indications
The CUSA® NXT Ultrasonic Surgical Aspirator is indicated for use in surgical procedures where fragmentation, emulsification and aspiration of soft and hard (e.g. bone) tissue is desirable, including Neurosurgery, Gastrointestinal and affiliated organ surgery, Urological surgery, Plastic and Reconstructive surgery, General surgery, Orthopedic surgery, Gynecological surgery, Thoracic surgery, Laparoscopic surgery and Thoracoscopic surgery.
Sales & Marketing EMEA Integra LifeSciences Services (France) SAS Immeuble Séquoia 2 97 allée Alexandre Borodine Parc technologique de la Porte des Alpes 69800 Saint Priest FRANCE Phone +33 (0)4 37 47 59 00 Fax +33 (0)4 37 47 59 99 emea.info@integralife.com integralife.eu
Contraindications
Do not use the CUSA® NXT ultrasonic surgical aspirator for other indications than the ones specified in the instructions for use.
Customer Services
Integra LifeSciences (Ireland) Ltd IDA Business and Technology Park Tullamore, Offaly, Irland
International: +33 (0)4 37 47 59 50 +33 (0)4 37 47 59 25 (Fax) csemea@integralife.com France: +33 (0)4 37 47 59 10 +33 (0)4 37 47 59 29 (Fax) custsvcfrance@integralife.com United Kingdom: +44 (0)1 26z4 345 780 +44 (0)1 264 363 782 (Fax) custsvcuk@integralife.com Germany: +49 (0) 2102 5535 6200 +49 (0)2 102 5536 636 (Fax) custsvcgermany@integralife.com Benelux: +32 (0)2 257 4130 +32 (0)2 253 2466 (Fax) custsvcbenelux@integralife.com Switzerland: +41 (0)2 27 21 23 30 +41 (0)2 27 21 23 99 (Fax) custsvcsuisse@integralife.com
BSI
Availability of these products might vary from a given country or region to another, as a result of specific local regulatory approval or clearance requirements for sale in such country or region. Always refer to the appropriate instructions for use for complete clinical instructions. Non contractual document. The manufacturer reserves the right, without prior notice, to modify the products in order to improve their quality. WARNING: Applicable laws restrict these products to sale by or on the order of a physician. SaberTip is a trademark of Integra LifeSciences Corporation or its subsidiaries. CUSA, Selector, Integra and the Integra logo are registered trademarks of Integra LifeSciences Corporation or its subsidiaries in the United States and/or other countries. Please read carefully the instructions for use. Products mentioned in this document are CE class I, IIb or III devices. Please contact Integra customer service should you need any additional information on devices classification. All the medical devices mentioned on this document are CE marked according to European council directive 93/42/EEC on medical devices and its relatives, unless specifically identified as “NOT CE MARKED”. Last revision date:07/2015. ©2015 Integra LifeSciences Corporation. All rights reserved. 0334043-1-EN
Document for use in Europe, Middle-East and Africa Only