Operation Manual
15 Pages
Preview
Page 1
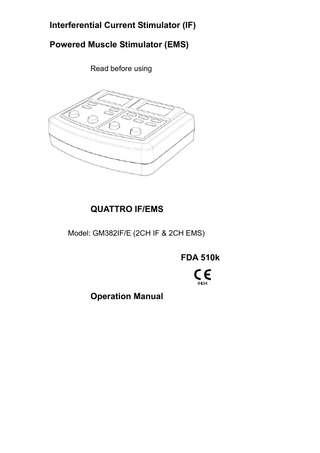
Interferential Current Stimulator (IF) Powered Muscle Stimulator (EMS) Read before using
QUATTRO IF/EMS Model: GM382IF/E (2CH IF & 2CH EMS)
FDA 510k
Operation Manual
TABLE CONTENTS GENERAL DESCRIPTION
1
SYSTEM COMPONENTS
1
WARRANTY
1
INDICATIONS AND CONTRAINDICATIONS
1
WARNINGS AND PRECAUTIONS
2
DANGER
4
ABOUT THE DEVICE
4
THE DEVICE CONTROLS
5
ATTACHING THE LEAD WIRES
6
ELECTRODE SELECTION AND CARE
6
TIPS FOR SKIN CARE
7
CONNECTING THE DEVICE
7
BATTERY INFORMATION
8
CHANGING THE BATTERY
8
9-VOLT DC ADAPTOR
8
CLEANING FOR YOUR DEVICE
8
TROUBLESHOOTING
9
TECHNICAL SPECIFICATIONS
10
GENERAL DESCRIPTION Electric stimulation has proven its high value as a method of pain therapy and is a great help to the experienced therapist. With some indications, physicians can prescribe a unit to patients for the use at home. This series of device is the combination of two units with dual-Channel electric stimulator for active treatment application, which is equipped with a Liquid Crystal Display indicating operation modes and output as well as an 8-bit micro computer for controlling the system. The electronics of the unit create electric impulses, the intensity duration, frequency per second and modulation of these impulses can be adjusted through the button or knob. SYSTEM COMPONENTS Your device may include the following components or accessories: z Unit z Carrying case z Lead wires / Electrodes z 6 x UM-3/AA size1.5V batteries. z Adaptor z Operation Manual WARRANTY This device carries a one-year warranty from the date of purchase. The warranty applies to the device and necessary parts and labor relating thereto. The distributor reserves the right to replace or repair the unit at their discretion. The warranty does not apply to damage resulting from failure to follow the operating instructions, accidents, abuse, alterations or disassembly by unauthorized individuals. INDICATIONS AND CONTRAINDICATIONS Read the operation manual before using the device. Federal law (USA) restricts this device for sale by or on the order of a physician. Observe your physician’s precise instructions and let him show you where to apply the electrodes. For a successful therapy, the correct application of the electrodes is an important factor. Carefully write down the settings your physician recommended.
-1-
The following statements are available for IF operation function Indications for use: This device may be used, with a physician’s prescription, for the symptomatic relief and management of chronic (long term) pain and for the treatment of postoperative or posttraumatic pain. CONTRAINDICATIONS z Patients with implanted electronic devices [for example, a pacemaker] or metallic implants should not undertake IF treatment without first consulting a physician. z Any electrode placement that applies current to the carotid sinus (neck) region. z Any electrode placement that causes current to flow trans-cerebrally. (through the head) z The use whenever pain symptoms are undiagnosed, until etiology is determined. The following statements are available for EMS operation function Indications for use z Relaxation of muscle spasms. z Prevention or retardation of disuse atrophy. z Increasing local blood circulation. Muscle re-education. z Maintaining or increasing range if motion. z Muscle re-education. z Immediate post-surgical stimulation of calf muscle to prevent venous thrombosis. Contraindications Powered muscle stimulators should not be used on patients with cardiac demand pacemakers. WARNINGS AND PRECAUTIONS The following statements are for IF operation function z z z
Warnings The device must be kept out of reach of children. The safety of device for use during pregnancy or delivery has not been established. Do not place electrodes on front of the throat. This may result is spasms of the laryngeal and pharyngeal muscles.
-2-
z z z z z z z
z z z z z z
Do not place the electrodes over the carotid nerve. The device is not effective for pain of central origin (headaches). The device may interfere with electronic monitoring equipment (such as ECG monitors and ECG alarms). Electrodes should not be placed over the eyes, in the mouth, or internally. These devices have no curative value. The devices should be used only under the continued supervision of a physician. IF is a symptomatic treatment and as such suppresses the sensation of pain which would otherwise serve as a protective mechanism. Precautions/adverse Reactions Isolated cases of skin irritation may occur at the site of electrode placement following long-term application. Stimulation should be stopped and electrodes removed until the cause of the irritation can be determined. Effectiveness is highly dependent upon patient selection by a person qualified in the management of pain patients. If the device treatment becomes ineffective or unpleasant, stimulation should be discontinued until reevaluation by a physician/clinician. Always turn the device off before applying or removing electrodes. Skin irritation and electrode burns are potential adverse reactions.
The following statements are for EMS operation function z z z z z z z z z
Warnings The long-term of chronic electrical stimulation are unknown. Stimulation should not be applied over the carotid sinus nerves, particularly in patients with a known sensitivity to the carotid sinus reflex. Stimulation should not be applied over the neck or mouth. Severe spasm of the laryngeal and pharyngeal muscles may occur and the contractions may be strong enough to close the airway or cause difficulty in breathing. Stimulation should not be applied trans-thoracically in that the introduction of electrical current into the heart may cause cardiac arrhythmias. Stimulation should not be applied transthoracically. Stimulation should not be applied over swollen, infected, or inflamed areas or skin eruptions, e.g. phlebitis, thrombophlebitis, varicose veins, etc. Stimulation should not be applied over, or in proximity to, cancerous lesions. Stimulation should not be applied trans-cerebrally (across the head). EMS devices should be used only under the continued supervision of a physician.
-3-
Precautions/adverse Reactions Safety of powered muscle stimulators for use during pregnancy has not been established. z Caution should be used for patients with suspected or diagnosed heart problems. z Caution should be used for patients with suspected or diagnosed epilepsy. z Caution should be used in the presence of the following: a. When there is a tendency to hemorrhage following acute trauma of fracture; b. Following recent surgical procedures when muscle contraction may disrupt the healing process; c. Over the menstruating or pregnant uterus; and d. Over areas of the skin which lack normal sensation. z Some patients may experience skin irritation or hypersensitivity due to the electrical stimulation or electrical conductive medium. The irritation can usually be reduced by using an alternate conductive medium, or alternate electrode placement. z Electrode placement and stimulation settings should be based on the guidance of the prescribing practitioner. z Powered muscle stimulators should be kept out of the reach of children. z Power muscle stimulators should be used only with the leads and electrodes recommended for use by the manufacturer. Skin irritation and burns beneath the electrodes have been reported with the use of powered muscle stimulators. z
DANGER The device does not have AAP/APG protection. Explosion hazard is possible if used in the presence of explosives, flammable materials or flammable anesthetics. Caution should be used when applying the device to patients suspected of having heart disease. Further clinical data is needed to show if there are adverse side effects on those with heart disease. ABOUT THE DEVICE Your device offers two controllable output channels for each stimulation unit. This device creates electrical impulses whose amplitude, duration, and modulation can be altered with the controls or buttons.
-4-
Model : GM382IF/E – 2 CH IF and 2 CH EMS
THE DEVICE CONTROLS General Basically the controls of device are available for different model as the following indication: Operation function Identification Mode Time Rate ∆∇ Width∆∇ ∆∇ SET ENT E (EMS) √ √ ± ± √ √ √ IF √ √ √ ± ± ± ± Intensity The intensity knobs located on the front of the unit for the strength adjustment of the stimulation and also function as ON/OFF controls. Mode The Mode key is used to select / set the type of treatment utilized. The available stimulation modes for each unit are as indicated in the following table: Identification Function as Available mode S (Synchronous), C (constant), E EMS A (Alternation) C (Constant), A (Auto sweep),S(Frequency IF Interferential Shift)
-5-
PS. IF has nine modes, please refer to its specification. TIME Treatment Time of device can be pre-select / set with Time key. There are four programs fixed duration of 15, 30, 45, 60 minute and continue. Press the key until engaged in position desired. 15 min : 15 min icon light on. 30 min : 15, 30 min icons light on. 45 min : 15,30 and 45 icons light on 60 min : 15, 30 and 60 min icons light on. Continue : the timer icon become empty. RATE ▲▼ The pulse Rate key regulates the number of pulse per second for both channels. SET/ENT The SET/ENT keys are used to select the type of treatment utilized. The type of treatment utilized includes cycle On time, On Ramp time, cycle Off time and Pulse Rate. The selected parameter will flash during adjust. ▲▼ The ▲▼ keys regulate the number of On, Ramp, Off and Pulse Rate value. ATTACHING THE LEAD WIRES The lead wires provided with the device insert into the jack sockets located on rear of the unit. Holding the insulated portion of the connector, push the plug end of the wire into one of the jacks. After connecting the wires to the stimulator, attach each wire to an electrode. Lead wires provided with the device are compliant with mandatory compliance standards set for the by FDA. Note: Use carefully when you plug and unplug the wires. Pulling on the lead wire instead of its insulated connector may cause wire breakage. Caution: Never insert the plug of the lead wire into an AC power supply socket. ELECTRODE SELECTION AND CARE Your physician/practitioner should decide which type of electrode is best for your condition. Follow application procedures outlined in electrode packaging to maintain stimulation and prevent skin irritation. The electrode packaging will provide instruction for care, maintenance and proper storage of your electrodes. Be sure to use the electrodes provided by the manufacturer and/or the similar FDA legally marketed electrode in particular the same cross section area.
-6-
TIPS FOR SKIN CARE Good skin care is important for comfortable use of your device. z Always clean the electrode site with mild soap and water solution, rinse well, and blot dry thoroughly prior to any electrode application. z Any excess hair should be clipped, not shoved, to ensure good electrode contact with the skin. z You may choose to use a skin treatment or preparation that is recommended by your physician. Apply, let dry, and apply electrode as directed. This will both reduce the chance of skin irritation and extend the life of your electrodes. z Avoid excessive stretching of the skin when applying electrodes, this is best accomplished by applying the electrode and smoothly pressing it in place from the center outward. z When removing electrodes, always remove by pulling in the direction of hair growth. z It may be helpful to rub skin lotion on electrode placement area when wearing electrodes. CONNECTING THE DEVICE 1. Prepare the Skin Prepare the skin as previously discussed and according to instructions provided with your electrodes. Before attaching the electrodes, identify the area in which your physician/practitioner has recommended for electrode placement. 2. Connect lead wires to the electrodes Connect the lead wires to the electrodes before applying the electrodes to the skin. Note: Be sure all intensity controls for every Channel 1 and 2 of each unit are turned to the “OFF” position. 3. Place Electrodes on Skin Place the electrodes on the skin as recommended by your clinician. 4. Insert Lead Wire Connector to device Plug end of lead wire into the channel output receptacle to be used, pushing plug in as far as it will go. 5. Select Treatment Settings Check and be sure your unit is still set to the proper settings recommended by your physician/practitioner. 6. Adjusting Channel Intensity Control Locate the intensity control knob at the front of the unit. Slowly turn the intensity control knob for the channels to be used clockwise until you reach the intensity
-7-
recommended by your medical professional. Always start with the lowest step and increase slowly. If the stimulation levels are uncomfortable or become uncomfortable, reduce the stimulation amplitude to a comfortable level or cease stimulation and contact your physician/practitioner if problems persist. BATTERY INFORMATION The 6 x 1.5-volt UM-3/AA size disposable batteries are provided with your unit. When the low battery mark light on the LCD the battery has become too weak to power the unit and it is time to change the battery. At this point, the unit will shot off until a fresh battery is inserted. CHANGING THE BATTERIES When the low battery mark light is on the LCD panel, the batteries should be replaced with the fresh batteries. 1. Remove the battery Cover by pressing this cover and sliding down until it is completely removed from the unit this will reveal the battery compartment. 2. Remove the discharged batteries from the device. 3. Place new batteries in compartment. Note the proper polarity alignment indicated on the battery and the compartment. 9-Volt DC Adaptor Because the current consumption is large for the device, it is recommended to use the 9V DC adaptor. Plug the DC plug into the DC jack of the unit, and plug the adaptor into your 110V wall outlet. Please be sure you are using the correct polarity of DC plug. Due to the high output of IF, the battery may run out of power very soon; therefore the 9-Volt DC adaptor is recommended. CLEANING FOR YOUR DEVICE Your device may be cleaned by wiping gently with a damp cloth moistened with mild soap and water. Never immerse the device in water or other liquids. Wipe lead wires with a damp cloth as above if they become soiled. To properly store the device extended period of time, remove the battery from the unit. Put the unit and accessories in the carrying case and store in a cool dry location.
-8-
TROUBLESHOOTING If the device does not function properly: 1. Make sure the battery is properly installed or replace battery. Be sure to observe proper polarity markings when replacing the battery. If the low battery mark light is on the LCD pane when the unit is turned on, replace the battery and check again. 2. If the intensity has been adjusted and there is no stimulation, check that the leas wires are properly connected and the electrodes are in place. If the unit appears to be functioning and no stimulation, the lead wires or electrodes may need to be replaced. 3. If the battery appears to be charged and the unit is not functioning, turn both intensity Control Knobs to the OFF position (counter clockwise) for about 7 sec. Then gradually turn the intensity Control Knob clockwise until stimulation is felt. If the device is still not working, turn off your device and contact your distributor. Note : For any problem, please contact your distributor. Do not try to repair this device by yourself.
-9-
TECHNICAL SPECIFICATIONS TECHNICAL SPECIFICATIONS FOR “IF” (Interferential) Channel: Pulse intensity:
Dual, isolated between channels. Adjustable 0-70mA peak into 500 ohm load each channel, constant current Carrier Frequency: 4000Hz fixed (CH1) Modulating Frequency: 4004-4160Hz Adjustable (CH2) IF Frequency Mode: Constant Mode (C): 4-160 bps, Adjustable Auto Sweep (A): 80-145 bps, 4-45 bps, 4 Set bps Frequency Shift (S): 1/1 abruptly shift, 6/6 abruptly shift, 6/6 ramped, 10/10 abruptly shift, 10/10 ramped Sweep Time: 15 seconds Frequency Shift Percent: Frequency shifts from 30% below set frequency to 60% above and return to 30% below set. Output Configuration: Quanta polar (4 electrodes) Wave form: Symmetrical balanced Sine wave Interference Pulse Freq: 4-160 bps, Adjustable 4 bps/step Pulse Duration: 125µs maximum Patient Compliance meter: Shows the treatment times Patient Lock Prevents the patient from changing any of the set parameters by the physician/practitioner. Timer: 15, 30, 45, 60 minute or Continue selectable LCD: Shows ,modes, bps rate, abrupt/ramp, timer and channels Max charge per Pulse: 17.5 Micro-coulombs Maximum Power Source: 9-Volt Battery (6x1.5V UM-3) or 9-Volt adaptor Tolerance: +/-10% **** bps: number of pulses per second
Output Parameters There are nine modes: Five modes w/frequency shift. Three modes W/Auto sweep, and one mode fully adjustable.
- 10 -
Constant Mode (C) Maintains set pulse frequency. (SET: bps can be adjusted) Auto Sweep (A) It will modulate frequency between the range that is selected. For example, select 80-145 bps auto sweep model. The modulation range of pulse frequency is from 80 bps to 145 bps. The sweep-time is 15 seconds and repeat. There are three mode of Auto Sweep: 1. 80 – 145 bps 2. 4 – 45 bps 3. 4 – SET bps Frequency Shift (S) There are five modes Frequency Shift” 1. 1/1 abruptly shift: The pulse frequency varies from –30% to +60% of the set pulse frequency. One second at the lower frequency, and one second at the higher frequency. The transition is abrupt (square wave function). 2. 6/6 abruptly shift: The pulse frequency varies from –30% to +60% of the set pulse frequency. Six seconds at the lower frequency, and six seconds at the higher frequency. The transition is abrupt (square wave function). 3. 6/6 ramped shift: The pulse frequency sweeps from –30% to +60% of the set pulse frequency. Six seconds from the lower frequency modulate to the high frequency, and six seconds from the higher frequency modulate to the lower frequency. The transition is ramping (triangular wave function). 4. 10/10 abruptly shift: The pulse frequency varies from –30% to +60% of the set pulse frequency. Ten seconds at the lower frequency, and ten seconds at the higher frequency. The transition is abrupt (square wave function). 5. 10/10 ramped shift: The pulse frequency sweeps from –30% to +60% of the pulse frequency. Ten seconds from the lower frequency modulate to the high frequency, and ten seconds from the higher frequency modulate to the lower frequency. The transition is ramping (triangular wave function).
- 11 -
TECHNICAL SPECIFICATIONS FOR “E” (EMS, Powered Muscle Stimulator) Channel: Pulse amplitude:
Dual, isolated between channels Adjustable 0-100mA peak into 500 Ω load each channel, constant current Pulse Rate: 1Hz-160Hz (adjustable), 1Hz/step Pulse Width: 300µs fixed. Software ramp up feature: Pulse width ramp up when change mode Patient Compliance Shows the treatment times. Account by hours. counter: Patient Lock System: To prevent the user from mis-changing any fixed parameter by physician. Timer: 15, 30, 45, 60 minute or Continue selectable LCD: Shows modes, pulse rate, pulse width, timer, CH1/CH2. ON Ramp: Adjustable 1-8 seconds. 1sec/step. Cycle ON Time: Adjustable 1-30 seconds. 1 sec/step. Cycle OFF Time: Adjustable 1-30 seconds. 1 sec/step. Function Modes: S: Synchronous. C: Constant. A: Alternation. Wave Form: Asymmetrical Bi-Phasic square pulse. Max charge per pulse: 30 micro-coulombs maximum Voltage: 0-110 Volt (Open Circuit). Power Source: 9-Volt Battery (6 x 1.5V UM-3/AA size) or 9-Volt adaptor All electrical specifications are ±10% 500Ω load.
- 12 -