Operators Manual
48 Pages
Preview
Page 1
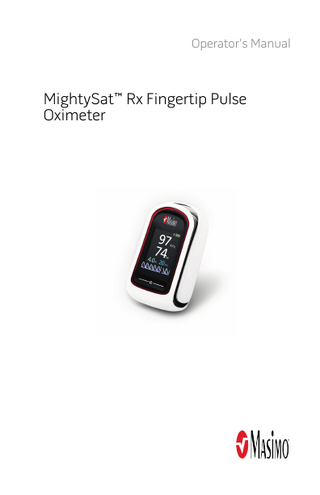
Operator's Manual
MightySat™ Rx Fingertip Pulse Oximeter
For Sale in the USA These operating instructions provide the necessary information for proper operation of all models of the MightySat™ Rx Fingertip Pulse Oximeter. There may be information provided in this manual that is not relevant for your system. General knowledge of pulse oximetry and an understanding of the features and functions of MightySat Rx are prerequisites for its proper use. Do not operate MightySat Rx without completely reading and understanding these instructions. Notice: Purchase or possession of this device does not carry any express or implied license to use with replacement parts which would, alone or in combination with this device, fall within the scope of one of the relating patents. CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. Note: Cleared Use Only: The device and related accessories are cleared by the Food and Drug Administration (FDA) for noninvasive patient monitoring and may not be used for any processes, procedures, experiments or any other use for which the device is not intended or cleared by the applicable regulatory authorities, or in any manner inconsistent with the directions for use or labeling. For professional use. See directions for use for full prescribing information, including indications, contraindications, warnings, precautions, and adverse events. Masimo Corporation 52 Discovery Irvine, CA 92618, USA Tel.: 949-297-7000 Fax.: 949-297-7001 www.masimo.com
3148433
MEDICAL ELECTRICAL EQUIPMENT WITH RESPECT TO ELECTRIC SHOCK, FIRE AND MECHANICAL HAZARDS ONLY IN ACCORDANCE WITH ANSI/AAMI ES 60601-1:2005, CAN/CSA C22.2 No. 60601-1:2008, and applicable Particular, (ISO 80601-2-61:2011) and related Collateral (IEC 60601-1-11:2010) Standards for which the product has been found to comply by Intertek.
Patents: www.masimo.com/patents.htm Masimo, , Signal Extraction Technology, SET, PVi, and Signal I.Q. are federally registered trademarks of Masimo Corporation. MightySat is a trademark of Masimo Corporation. All other trademarks and registered trademarks are property of their respective owners. © 2018 by Masimo Corporation.
www.masimo.com
1
Masimo
Contents About this Manual ------------------------------------------------------------------------------------------ 5 Product Description and Indications ---------------------------------------------------------------------7 Product Description --------------------------------------------------------------------------------------7 Indications for Use---------------------------------------------------------------------------------------7 Safety Information, Warnings, and Cautions ----------------------------------------------------------- 9 Safety Warnings and Cautions ------------------------------------------------------------------------ 9 Performance Warnings and Cautions ---------------------------------------------------------------- 9 Cleaning, Disinfecting, Service Warnings and Cautions ----------------------------------------- 11 Compliance Warnings and Cautions ---------------------------------------------------------------- 12 Technology Overview -------------------------------------------------------------------------------------- 13 Signal Extraction Technology® (SET®) ------------------------------------------------------------ 13 Operation ---------------------------------------------------------------------------------------------------- 17 MightySat Rx Features -------------------------------------------------------------------------------- 17 Installing the AAA Batteries ------------------------------------------------------------------------- 18 Using MightySat Rx ------------------------------------------------------------------------------------ 18 Turning off MightySat Rx ----------------------------------------------------------------------------- 19 Using the Touchpad ---------------------------------------------------------------------------------- 20 Navigating the Menu ---------------------------------------------------------------------------------- 21 Connecting to a Smart Device via Bluetooth (Optional) ---------------------------------------- 21 Bluetooth Connection Overview --------------------------------------------------------------------- 22 Cleaning, Disinfecting, and Service --------------------------------------------------------------------- 23 Cleaning and Disinfecting MightySat Rx ----------------------------------------------------------- 23 Service --------------------------------------------------------------------------------------------------- 24 Troubleshooting ---------------------------------------------------------------------------------------- 24 Product Support ---------------------------------------------------------------------------------------- 25 Specifications----------------------------------------------------------------------------------------------- 29 Display Range ------------------------------------------------------------------------------------------- 29 Performance Specifications -------------------------------------------------------------------------- 29 SpO2 Performance Specifications ------------------------------------------------------------------ 30 Battery Life ---------------------------------------------------------------------------------------------- 31 Environment -------------------------------------------------------------------------------------------- 31 www.masimo.com
3
Masimo
MightySat Rx
Contents
Physical Characteristics ------------------------------------------------------------------------------ 31 Compliance --------------------------------------------------------------------------------------------- 32 Bluetooth LE Wireless Technology Information -------------------------------------------------- 33 Guidance and Manufacturer's Declaration - Electromagnetic Emissions -------------------- 34 Guidance and Manufacturer's Declaration - Electromagnetic Immunity -------------------- 35 Recommended Separation Distances -------------------------------------------------------------- 37 Symbols-------------------------------------------------------------------------------------------------- 38 Citations ------------------------------------------------------------------------------------------------- 40 Index --------------------------------------------------------------------------------------------------------- 43
www.masimo.com
4
Masimo
About this Manual Do not operate the MightySat™ Rx Fingertip Pulse Oximeter without completely reading and understanding the instructions. Always use the MightySat Rx precisely in accordance with the directions in this manual, including site selection and sensor placement. Failure to follow all of the directions in this manual could lead to inaccurate measurements. Read and follow any warnings, cautions, and notes presented throughout this manual. The following are explanations of warnings, cautions, and notes. A warning is given when actions may result in a serious outcome to the patient or user (for example, injury, serious adverse effect, or death). WARNING: This is an example of a warning statement. A caution is given when any special care is to be exercised by the patient or user to avoid injury to the patient, damage to this instrument, or damage to other property. CAUTION: This is an example of a caution statement. A note is given when additional general information is applicable. Note: This is an example of a note.
www.masimo.com
5
Masimo
Product Description and Indications Product Description The MightySat™ Rx Fingertip Pulse Oximeter is intended as a noninvasive device that measures and displays arterial oxygen saturation (SpO2), Pulse Rate (PR), Perfusion Index (Pi), and optional Pleth Variability Index (PVi®). The following key features are available for the MightySat Rx: •
Masimo SET® technology for SpO2 and pulse rate monitoring in motion and low perfusion environments.
•
Optional Bluetooth® LE wireless technology for the wireless transfer of patient data to smart devices.
The MightySat™ Rx Fingertip Pulse Oximeter is available in the following versions: Product Versions
Features
MightySat Rx
Intended to measure and display arterial oxygen saturation (SpO2), Pulse Rate (PR), and Perfusion Index (Pi).
MightySat Rx, Bluetooth LE
Intended to measure and display arterial oxygen saturation (SpO2), Pulse Rate (PR), and Perfusion Index (Pi). Optional Bluetooth LE radio is intended to transfer of parameter data to a compatible smart device.
MightySat Rx, Intended to measure and display arterial oxygen saturation (SpO2), Bluetooth LE and PVi Pulse Rate (PR), Perfusion Index (Pi), and optional Pleth Variability Index (PVi). Optional Bluetooth LE radio is intended for transfer of parameter data to a compatible smart device.
Indications for Use The MightySat™ Rx Fingertip Pulse Oximeter is indicated for the noninvasive spot checking of functional oxygen saturation of arterial hemoglobin (SpO2) and pulse rate (PR). The Masimo MightySat™ Rx Fingertip Pulse Oximeter is indicated for use with adult and pediatric patients during both no motion and motion conditions, and for patients who are well or poorly perfused in hospitals, hospital-type facilities, and mobile environments.
www.masimo.com
7
Masimo
Safety Information, Warnings, and Cautions Safety Warnings and Cautions •
WARNING: Do not use MightySat Rx during magnetic resonance imaging (MRI) or in an MRI environment.
•
WARNING: Do not place MightySat Rx or accessories in any position that might cause it to fall on the patient.
•
WARNING: Do not use MightySat Rx during defibrillation.
•
WARNING: Do not use MightySat Rx during electrosurgery.
•
WARNING: Do not use MightySat Rx in the presence of flammable anesthetics or other flammable substances, oxygen-enriched environments, or nitrous oxide to avoid the risk of explosion.
•
WARNING: Only use the MightySat Rx to the secure it to the finger. Excessive pressure to a finger can cause skin damage.
•
WARNING: Check the sensor site every hour to ensure adequate circulation, skin integrity, and sensor alignment. Skin damage, pressure necrosis, or inaccurate readings may result.
•
WARNING: Do not leave the MightySat Rx unattended around children. Small items such as the battery door, battery, and lanyard may become choking hazards.
•
WARNING: Do not use the lanyard during activities where it may become wrapped around the neck. Strangulation may occur.
•
CAUTION: Do not use the MightySat Rx near devices that are sensitive to magnets. The magnet provided with the MightySat Rx could interfere with the proper operation of the device.
•
Note: The maximum skin surface temperature is measured to be less than 41°C (106°F) in a 35°C (95°F) environment. This was verified by measuring the skin interface temperature with MightySat Rx operating under reasonable worst-case conditions.
Performance Warnings and Cautions •
WARNING: MightySat Rx is not an apnea monitor and should not be used for arrhythmia analysis.
•
WARNING: MightySat Rx should not be used as the sole basis for medical decisions. It must be used in conjunction with clinical signs and symptoms.
•
WARNING: Do not self-diagnose or self-medicate on the basis of the measurements. Always consult your doctor.
•
WARNING: Do not use MightySat Rx for continuous monitoring. It is intended for spot-check use only. No alarms are provided to support continuous monitoring.
•
WARNING: Do not use MightySat Rx if it appears or is suspected to be damaged. Damage to internal parts can result in no or inaccurate readings.
www.masimo.com
9
Masimo
MightySat Rx
Safety Information, Warnings, and Cautions
•
WARNING: Do not repair, open, or modify MightySat Rx. Damage to internal parts can result in no or inaccurate readings.
•
WARNING: Do not use the MightySat Rx if the internal parts have been exposed to liquids. Damage to the internal parts may result in no or inaccurate readings.
•
WARNING: SpO2 is empirically calibrated in healthy adult volunteers with normal levels of carboxyhemoglobin (COHb) and methemoglobin (MetHb).
•
WARNING: Avoid the following conditions to minimize the risk of inaccurate SpO2 readings: •
Improper MightySat Rx placement or alignment
•
Elevated levels of COHb and MetHb: High levels of COHb or MetHb may occur with a seemingly normal SpO2. When elevated levels of COHb or MetHb are suspected, laboratory analysis (CO-Oximetry) of a blood sample should be performed.
•
Intravascular dyes such as indocyanine green or methylene blue
•
Externally applied coloring and texture such as nail polish, acrylic nails, glitter, etc.
•
Patients attached to a high pressure cuff
•
Placing the MightySat Rx sensor on any extremity with an arterial catheter or blood pressure cuff
•
Elevated levels of bilirubin
•
Severe anemia
•
Venous congestion
•
Venous pulsation
•
Extremely low arterial perfusion
•
Excessive motion
•
CAUTION: Properly apply and avoid using the MightySat Rx under high ambient light sources, fluorescent lights, infrared heating lamps and direct sunlight to minimize interference that may result in no or inaccurate readings.
•
CAUTION: Keep the MightySat Rx away from electrical equipment that emits radio frequencies to minimize radio interference. Radio interference may result in no or inaccurate readings.
•
CAUTION: When using MightySat Rx with a smart device, keep both devices within the recommended range of each other (see Specifications for details); moving outside of this range may cause a loss in connection with the smart device.
•
CAUTION: When using MightySat Rx with a smart device, relocate the devices away from sources that may interfere with the Bluetooth connection. The presence of other devices that may create radio frequency interference (RFI) may result in loss of Quality of Service (see Specifications for details) of the Bluetooth connection. Devices that may cause RFI include but are not limited to the following: electrocautery equipment, diathermy equipment, other cellular telephones, wireless PC and tablets, pagers, RFID devices, MRI, and electromagnetic security systems.
www.masimo.com
10
Masimo
MightySat Rx
Safety Information, Warnings, and Cautions
•
CAUTION: Do not attempt to remanufacture, recondition, or recycle MightySat Rx as these processes may damage the internal parts. Damage to internal parts can result in no or inaccurate readings.
•
Note: The MightySat Rx display may be difficult to view when exposed to direct sunlight or bright lights.
•
Note: Do not assess the accuracy of the MightySat Rx using a functional tester. A functional tester should only be used to check if a unit is working properly.
•
Note: The MightySat Rx display will shut off automatically if there are no readings.
Cleaning, Disinfecting, Service Warnings and Cautions •
WARNING: Properly use and dispose of Alkaline batteries or they may leak or explode.
•
WARNING: Remove alkaline batteries when the MightySat Rx will not be in use for more than 30 days to avoid damage to the device due to batteries that may leak.
•
WARNING: Replace both batteries at the same time to avoid mixing fully and partially charged batteries. These actions may cause the batteries to leak; resulting in possible damage to the device.
•
CAUTION: Use only AAA alkaline batteries. Use of non-alkaline batteries may affect the accuracy of the battery status indicator.
•
CAUTION: Only perform maintenance procedures specifically described in the manual; otherwise, return MightySat Rx for servicing. Improper maintenance may result in damage to the internal parts. Damage to internal parts may result in no or inaccurate readings.
•
CAUTION: Thoroughly clean and low level disinfect the MightySat Rx before applying it to on a new patient.
•
CAUTION: Do not clean MightySat Rx with any chemical other than those specified in Cleaning, Disinfecting, and Service on page 23. These substances may affect the device’s materials and damage internal parts.
•
CAUTION: Do not submerge MightySat Rx in any cleaning solution or attempt to sterilize by autoclave, irradiation, steam, gas, ethylene oxide or any other method. This will seriously damage the device.
•
CAUTION: Do not use undiluted bleach (5% - 5.25% sodium hypochlorite) or any cleaning solution other than those recommended in Cleaning, Disinfecting, and Service on page 23 of this manual. Permanent damage to MightySat Rx may occur if other unspecified solutions are used.
•
CAUTION: Never submerge MightySat Rx in water or any other liquid solution this may cause permanent damage to the MightySat Rx.
www.masimo.com
11
Masimo
MightySat Rx
Safety Information, Warnings, and Cautions
Compliance Warnings and Cautions •
WARNING: Any changes or modifications not expressly approved by Masimo shall void the warranty for this equipment and could void the user’s authority to operate the equipment.
•
CAUTION: Comply with local laws in the disposal of the instrument and/or its accessories, including batteries.
•
CAUTION: This device is not intended to be operated in the home environment.
•
CAUTION: This device has not been evaluated for use in aircrafts.
•
Note: When using MightySat Rx with a device with wireless features, consideration should be taken to local government frequency allocations and technical parameters to minimize the possibility of interference to/from other wireless devices.
•
Note: In accordance with international telecommunication requirements, the frequency band of 2.4 GHz is only for indoor usage to reduce potential for harmful interference to co-channel mobile satellite systems.
•
Note: This device complies with Part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) This device may not cause harmful interference, and (2) this device must accept any interference received, including interference that may cause undesired operation.
•
Note: This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to part 15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference in a residential installation. This equipment generates, uses, and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to radio communications. However, there is no guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interference to radio or television reception, which can be determined by turning the equipment off and on, the user is encouraged to try to correct the interference by one or more of the following measures: •
Reorient or relocate the receiving antenna.
•
Increase the separation between the equipment and receiver.
•
Consult the dealer or an experienced radio/TV technician for help.
•
Note: This equipment has been tested and found to comply with the Class B limits for medical devices according to the EN 60601-1-2: 2007, Medical Device Directive 93/42/EEC. These limits are designed to provide reasonable protection against harmful interference in all establishments, including domestic establishments.
•
Note: This Class B digital apparatus complies with Canadian ICES-003.
www.masimo.com
12
Masimo
Technology Overview The following chapter contains general descriptions about parameters, measurements, and the technology used by Masimo products.
Signal Extraction Technology® (SET®) Masimo Signal Extraction Technology's signal processing differs from that of conventional pulse oximeters. Conventional pulse oximeters assume that arterial blood is the only blood moving (pulsating) in the measurement site. During patient motion, however, the venous blood also moves, causing conventional pulse oximeters to read low values, because they cannot distinguish between the arterial and venous blood movement (sometimes referred to as noise). Masimo SET® pulse oximetry utilizes parallel engines and adaptive filtering. Adaptive filters are powerful because they are able to adapt to the varying physiologic signals and/or noise and separate them by looking at the whole signal and breaking it down to its fundamental components. The Masimo SET® signal processing algorithm, Discrete Saturation Transform® (DST®), in parallel with Fast Saturation Transform (FST®), reliably identifies the noise, isolates it and, using adaptive filters, cancels it. It then reports the true arterial oxygen saturation for display on the monitor.
Masimo SET® DST This figure is for conceptual purposes only.
General Description for Oxygen Saturation (SpO2) Pulse oximetry is governed by the following principles: •
Oxyhemoglobin (oxygenated blood) and deoxyhemoglobin (non-oxygenated blood) differ in their absorption of red and infrared light (spectrophotometry).
•
The amount of arterial blood in tissue changes with your pulse (photoplethysmography). Therefore, the amount of light absorbed by the varying quantities of arterial blood changes as well.
www.masimo.com
13
Masimo
MightySat Rx
Technology Overview
Successful Monitoring for SpO2, PR and Pi Stability of the SpO2 readings may be a good indicator of signal validity. Although stability is a relative term, experience will provide a good feeling for changes that are artifactual or physiological and the speed, timing, and behavior of each. The stability of the readings over time is affected by the averaging time being used. The longer the averaging time, the more stable the readings tend to become. This is due to a dampened response as the signal is averaged over a longer period of time than during shorter averaging times. However, longer averaging times delay the response of the oximeter and reduce the measured variations of SpO2 and pulse rate.
Functional Oxygen Saturation (SpO2) The MightySat Rx is calibrated to measure and display functional oxygen saturation (SpO2): the amount of oxyhemoglobin expressed as a percentage of the hemoglobin that is available to transport oxygen. Note: Dyshemoglobins are not capable of transporting oxygen, but are recognized as oxygenated hemoglobins by conventional pulse oximetry.
General Description for Perfusion Index (Pi) The Perfusion Index (Pi) is the ratio of the pulsatile blood flow to the non-pulsatile or static blood in peripheral tissue. PI thus represents a noninvasive measure of peripheral perfusion that can be and noninvasively obtained from a pulse oximeter.
General Description for Pulse Rate (PR) Pulse rate (PR), measured in beats per minute (BPM) is based on the optical detection of peripheral flow pulse.
General Description for Pleth Variability Index (PVi) The Pleth Variability Index (PVi) is a measure of the dynamic changes in the Perfusion Index (Pi) that occur during the respiratory cycle. The calculation is accomplished by measuring changes in Pi over a time interval where one or more complete respiratory cycles have occurred. PVi is displayed as a percentage (0-100%). PVi may show changes that reflect physiological factors such as vascular tone, circulating blood volume, and intrathoracic pressure excursions. The utility of PVi has been evaluated in clinical studies [1-11]. Technical and clinical factors that may affect PVi include probe malposition, probe site, patient motion, skin incision, spontaneous breathing activity, lung compliance, open pericardium, use of vasopressors or vasodilators, low perfusion index, subject age, arrhythmias, left or right heart failure, and tidal volume [12-14].
www.masimo.com
14
Masimo
MightySat Rx
Technology Overview
Citations for Pleth Variability Index (PVi) 1.
2. 3.
4.
5. 6. 7. 8.
9. 10.
11. 12. 13. 14.
Cannesson M., Desebbe O., Rosamel P., Delannoy B., Robin J., Bastien O., Lehot J.J. Pleth Variability Index to Monitor the Respiratory Variations in the Pulse Oximeter Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the Operating Theatre. Br J Anaesth. 2008 Aug;101(2):200-6. Forget P, Lois F, de Kock M. Goal-Directed Fluid Management Based on the Pulse Oximeter-Derived Pleth Variability Index Reduces Lactate Levels and Improves Fluid Management. Anesth Analg. 2010 Oct;111(4):910-4. Zimmermann M., Feibicke T., Keyl C., Prasser C., Moritz S., Graf B.M., Wiesenack C. Accuracy of Stroke Volume Variation Compared with Pleth Variability Index to Predict Fluid Responsiveness in Mechanically Ventilated Patients Undergoing Major Surgery. Eur J Anaesthesiol. 2010 Jun;27(6):555-61. Desebbe O, Boucau C, Farhat F, Bastien O, Lehot JJ, Cannesson M. Anesth Analg. The Ability of Pleth Variability Index to Predict the Hemodynamic Effects of Positive End-Expiratory Pressure in Mechanically Ventilated Patients under General Anesthesia. 2010 Mar 1;110(3):792-8. Tsuchiya M., Yamada T., Asada A. Pleth Variability Index Predicts Hypotension During Anesthesia Induction. Acta Anesthesiol Scand. 2010 May;54(5):596-602. Loupec T., Nanadoumgar H., Frasca D., Petitpas F., Laksiri L., Baudouin D., Debaene B., Dahyot-Fizelier C., Mimoz O. Pleth Variability Index Predicts Fluid Responsiveness in Critically Ill Patients. Crit Care Med. 2011 Feb;39(2):294-9. Fu Q., Mi W.D., Zhang H. Stroke Volume Variation and Pleth Variability Index to Predict Fluid Responsiveness during Resection of Primary Retroperitoneal Tumors in Hans Chinese. Biosci Trends. 2012 Feb;6(1):38-43. Haas S., Trepte C., Hinteregger M., Fahje R., Sill B., Herich L., Reuter D.A. J. Prediction of Volume Responsiveness using Pleth Variability Index in Patients Undergoing Cardiac Surgery after Cardiopulmonary Bypass. Anesth. 2012 Oct;26(5):696-701. Byon H.J., Lim C.W., Lee J.H., Park Y. H., Kim H.S., Kim C.S., Kim J.T. Br. J. Prediction of fluid Responsiveness in Mechanically Ventilated Children Undergoing Neurosurgery. Anaesth 2013 Apr;110(4):586-91. Feissel M., Kalakhy R., Banwarth P., Badie J., Pavon A., Faller J.P., Quenot JP. Plethysmographic Variation Index Predicts Fluid Responsiveness in Ventilated Patients in the Early Phase of Septic Shock in the Emergency Department: A Pilot Study. J Crit Care. 2013 Oct;28(5):634-9. Yu Y., Dong J., Xu Z., Shen H., Zheng J. Pleth Variability Index-Directed Fluid Management in Abdominal Surgery under Combined General and Epidural Anesthesia. J Clin Monit Comput. 2014 Feb 21. Desgranges F.P., Desebbe O., Ghazouani A., Gilbert K., Keller G., Chiari P., Robin J.,Bastien O., Lehot J.J., Cannesson M. Br. J. Anaesth 2011 Sep;107(3):329-35. Cannesson M. Arterial pressure variation and goal-directed fluid therapy. J Cardiothorac Vasc Anesth. 2010 Jun;24(3):487-97. Takeyama M, Matsunaga A, Kakihana Y, Masuda M, Kuniyoshi T, Kanmura Y. Impact of Skin Incision on the Pleth Variability Index. J Clin Monit Comput 2011 Aug;25(4):215-21.
www.masimo.com
15
Masimo
Operation MightySat Rx Features
ID Description
Function
1
Enclosure Clip
Clip provided for ease of lanyard attachment.
2
Bluetooth Indicator (Optional)
Indicates when Bluetooth LE is enabled on the device.
3
Battery Status Indicator
Indicates the remaining relative life of the battery.
4
Display Screen
Display for measurements and indicators. Note: Numbers will dim when confidence in the value is low.
5
Waveform and SIQ or Pulse Bar
When the waveform option is turned on, the pleth waveform and the SIQ provides an indicator of the relative signal strength. When the waveform option is turned off, the pulse bar provides a blinking graphical indicator which corresponds to the pulse rate. Additionally, the relative signal strength is depicted by the height of the pulse bar.
6
Touchpad
www.masimo.com
User interface to allow for change of settings (see Using the Touchpad on page 20 in this manual).
17
Masimo
MightySat Rx
Operation
Installing the AAA Batteries The MightySat Rx requires two alkaline AAA batteries to operate. To install batteries, follow the instructions below: 1. 2. 3. 4.
5.
Place the MightySat Rx so that the display screen is facing downwards. Find the battery button on the front of the sensor pad. Push lightly on the battery button to release the battery cover and then remove the battery cover. Insert two new AAA alkaline batteries and match the orientation labels (+ and -). Note: MightySat Rx will not work if the batteries are inserted in the incorrect orientation. Once the batteries are correctly inserted, snap the battery door back onto the device. WARNING: Ensure that the battery door is intact before use. Note: MightySat Rx will turn on automatically when the device is opened so that the sensor pads are exposed as shown in the image below.
Using MightySat Rx To take readings with the MightySat Rx, follow the instruction below: Note: Before use, ensure batteries are correctly installed in the MightySat Rx.
1. 2.
3.
To open the MightySat Rx, squeeze the back portion of the device as shown in the image above. Once the sensor pads are exposed, insert a finger (non-dominant, ring finger) so the sensor LED is above the fingernail. Note: The display screen of the MightySat Rx should be facing upwards as depicted in the image above. Once the finger is correctly positioned, gently close the MightySat Rx by releasing the pressure on the back of the device. Note: Ensure the finger is correctly positioned for accurate measurements.
www.masimo.com
18
Masimo
MightySat Rx
Operation
4.
The tip of the finger should touch the finger stop as shown in the image below.
5.
Once the MightySat Rx is correctly closed on the finger, the MightySat Rx will display readings. Note: If no readings are displayed, see Troubleshooting on page 24 in this manual. WARNING: While on the finger, do not press the top of the device against any surface. WARNING: Do not attempt to secure the MightySat Rx to the finger using external pressure. The internal spring provides the correct pressure; additional pressure may cause inaccurate readings.
Turning off MightySat Rx The MightySat Rx turns off automatically after removing the finger from the device in the absence of device interaction or connection to a smart device.
www.masimo.com
19
Masimo
MightySat Rx
Operation
Using the Touchpad The Touchpad screen.
on MightySat Rx device is located below the display
Note: The display is not a touch screen. Action Description
Function
Touch
Tap and Select a menu item or action. release. Action Touch rotates the display clockwise. will be performed once finger is released.
Press and Hold
Press and hold. Enter and exit the Main Screen. Release finger once action has been performed.
Swipe
Touch and slide Scroll through all selectable menu options. (left, and right) and release.
www.masimo.com
20
Masimo
MightySat Rx
Operation
Navigating the Menu From the Main Screen, press and hold the Touchpad to access the Main Menu. Use the Touchpad Swipe gesture to scroll through the Main Menu Options. Use the Touch gesture to select the Main Menu Option. Use the same gestures to adjust settings. The Menu options are: Main Menu Options
Display Button
Description
Default Options
Back
Return to Main Screen.
N/A
N/A
Waveform
Allows the user to choose if the waveform will be displayed on the screen.
On
On or Off
Brightness
Change the brightness of the display screen.
100%
25%, 50%, 75%, and 100%
Bluetooth (Optional)
Allows the user to connect to a smart device via Bluetooth LE.
On
On or Off
About
Hardware and software information about the device including serial number, software version, and Bluetooth LE Mac Address.
N/A
N/A
Connecting to a Smart Device via Bluetooth (Optional) Note: Bluetooth LE is an optional feature available on specific versions of MightySat Rx for use with compatible smart devices. For a full list of compatible smart devices, see www.masimoprofessionalhealth.com.
www.masimo.com
21
Masimo
MightySat Rx
Operation
Bluetooth Connection Overview The MightySat Rx provides a Bluetooth LE wireless option to allow connection to a compatible smart device. The Bluetooth communication is only available to smart devices using the Masimo Professional Health App. When a Bluetooth connection is established the Bluetooth connected icon will appear.MightySat Rx can only communicate to a single smart device at one time to minimize the risk of unauthorized access. 1. 2. 3.
Turn on Bluetooth on MightySat Rx. See Navigating the Menu on page 21 of this manual for further instructions. Ensure the Bluetooth is enabled on the smart device. From your compatible smart device, do one of the following: •
4. 5.
6.
7.
For AndroidTM-powered devices, go to the Google PlayTM store.
• For Apple® devices, go to the App StoreSM. Search and download the “Masimo Professional Health” app. Launch the Masimo Professional Health app to connect the MightySat Rx with a compatible smart device. Note: The MightySat Rx requires the use of the Masimo Professional Health app to communicate to a compatible smart device. Confirm the correct connection by viewing the appropriate "Serial" number is displayed on the smart device. The Serial number can be found on the About screen, see Navigating the Menu on page 21 of this manual for information on accessing the About screen. Once MightySat Rx is connected to a smart device, confirm the readings on the MightySat Rx to the readings displayed on the Masimo Professional Health app are synchronized without a delay greater than 10 seconds. Note: A connection icon will appear on the MightySat Rx device when a Bluetooth connection has been established. Note: If the delay is greater than 10 seconds, move the MightySat Rx in closer proximity to the smart device or attempt to repeat the connection process. Note: To prevent unauthorized connection to the MightySat Rx, turn off the optional Bluetooth LE feature on the MightySat Rx. CAUTION: When using MightySat Rx (optional Bluetooth version) with a smart device, keep both devices within the recommended range of each other (see Specifications on page 29 for details); moving outside of this range may cause a loss in connection with the smart device. CAUTION: When using MightySat Rx (optional Bluetooth version) with a smart device, relocate the devices away from sources that may interfere with the Bluetooth connection. Interference may result in loss of Quality of Service (see Specifications on page 29 for details) of the Bluetooth connection.
www.masimo.com
22
Masimo
Cleaning, Disinfecting, and Service Cleaning and Disinfecting MightySat Rx WARNING: Before cleaning, read Cleaning, Disinfecting, Service Warnings and Cautions on page 11 in this manual. WARNING: Before cleaning, make sure the device is off and is not applied to a finger. CAUTION: Thoroughly clean and low level disinfect the MightySat Rx before applying it to on a new patient. Note: Before cleaning, remove the batteries and make sure the battery cover is re-attached correctly. To clean the MightySat Rx, follow the instructions below: •
Wipe each of the sensor pads and outer surfaces using a CaviWipes™ wipe twice or until the surfaces are free of any visible residue. Note: Pay particular attention to cracks, crevices, and hard to reach areas of the device.
•
Repeat the above cleaning step using a fresh wipe.
•
Allow the MightySat Rx to dry thoroughly before using again.
To conduct low level surface disinfection of the MightySat Rx, follow the instructions below: Note: Follow cleaning instructions prior to disinfecting the device. •
Visibly wet the sensor pads and outer surfaces using a soft cloth dampened with a 10% (1:10) chlorine bleach to water solution.
•
Allow the solution to sit for 10 minutes on the sensor pads before wiping them with a dry soft cloth.
•
Allow the MightySat Rx to dry thoroughly before using again.
Alternatively, the MightySat Rx can be disinfected using the same instructions above, except with CaviWipes with a 5 minute exposure time. The surfaces of the MightySat Rx have been tested to be chemically resistant to following solution(s): •
70% Isopropyl Alcohol
•
Cidex Plus (3.4% glutaraldehyde)
•
10% (1:10) chlorine bleach to water solution
•
Quaternary ammonium chloride solution wipe (CaviWipes™)
CAUTION: To avoid permanent damage to the MightySat Rx, do not use undiluted bleach (5% - 5.25% sodium hypochlorite) or any other cleaning solution not recommended.
www.masimo.com
23
Masimo