medtronic
Skeeter Ultra-Lite Oto-Tool Handpiece Instructions for Use rev B April 2022
Instructions for Use
212 Pages
Preview
Page 1
EN
EN
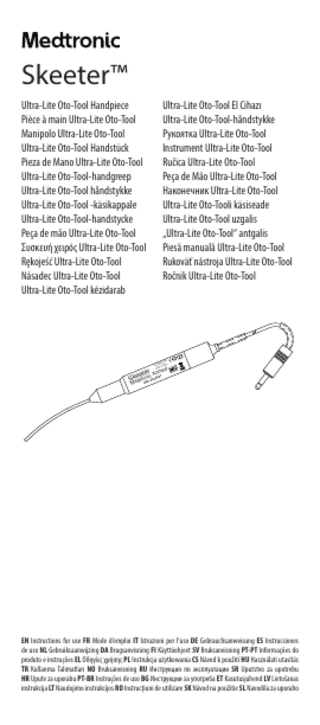
PHYSICIAN NOTE: Review the instructions for use for applicable information to be shared with the patient such as warnings, precautions, contraindications, and limitations of use regarding the device. This device is intended for use in combination with the IPC™ console or the XPS™ 3000 console for safe use and performance. Any other combination may result in a hazard to the patient. The use of components or devices of other manufacturers in conjunction with the Skeeter™ handpiece has not been verified. The performance characteristics of this device may be altered if components or devices of other manufacturers are used in conjunction with this device. The performance characteristics are verified for all Medtronic IPC™ and XPS™ 3000 consoles and related handpieces and tools, which are designed to be interchangeably connected with each other. For additional information regarding Skeeter™ handpiece use with the IPC™ console and the XPS™ 3000 console, including detailed operating instructions, electromagnetic information, and other technical specifications, refer to the IPC™ system and XPS™ 3000 system user’s guides. Device description The Skeeter™ handpiece and burs may be used with the IPC™ and XPS™ 3000 consoles via direct connection with the Skeeter™ handpiece connector. The lightweight Skeeter™ handpiece weighs 57 grams. The drill shaft diameter is approximately 2 mm and is angled approximately 20 degrees from the plane of the handpiece. The shaft angulation and small diameter maximize visualization of the surgical field during drill use. The Skeeter™ handpiece is designed for variable speed operation of up to high speed of 12,000 rpm. The Skeeter™ handpiece can be operated by a console with a foot pedal. However, when used with the XPS™ 3000 console, the handpiece plugs directly into the console and is operated using the XPS™ 3000 single function or multifunction foot pedal. The foot pedal allows variable speed operation of the handpieces ranging from very slow to high speed of 12,000 rpm. The Skeeter™ handpiece is used with the Oto-Flex bur. The Oto-Flex burs designed for use with the Skeeter™ handpiece are composed of a flexible stainless steel shaft with a bur and a PTFE bearing at one end and the Skeeter™ handpiece engagement at the other. The inside of the PTFE bearing is lightly coated with silicone spray to reduce operating friction. Color Coding For ease of identification of size, all Oto-Flex burs are color coded. Diamond burs are further differentiated from carbide burs by a white band on the shaft of each diamond bur. A color code chart is conveniently provided in the base of the Skeeter™ sterilization tray. The bur size identification color code is as follows: Color
Bur Size
Color
Bur Size
Color
Bur Size
Violet
0.5 mm
Yellow
0.8 mm
Brown
1.8 mm
Blue
0.6 mm
Orange
1.0 mm
Red
2.3 mm
Green
0.7 mm
Gray
1.4 mm
Black
Specialty
Intended use The Skeeter™ Ultra-Lite handpiece is intended to surgically remove bone from ear canal and middle ear structures. Indications for use The Skeeter™ Ultra-Lite handpiece is used to surgically remove bone from the ear canal and middle ear structures. It is specifically used in middle ear surgical procedures, including stapes footplate surgery. The Skeeter™ handpiece is intended to be used on patients undergoing surgical procedures of the middle ear. Intended clinical benefit The Skeeter™ handpiece does not provide any clinical benefit for the patient. Training requirements This device is intended for use by physicians trained in the procedures described in the indications for use. Contraindications None are known. Warnings • It is important that the Skeeter™ handpiece operator be familiar with the system user’s guide, its precautions, procedures and safety issues. • Do not use the Skeeter™ handpiece in the presence of flammable anesthetics. Avoid potential ignition or explosion of gases. 1
EN
•
Do not attach any component or accessory other than Medtronic approved components to the Skeeter™ handpiece as this may result in electrical shock, component damage, substandard performance, increased emissions, or decreased immunity. • Disconnect power to the Skeeter™ handpiece before cleaning the unit to avoid electrical shock. • This medical device complies with EN 60601-1-2 safety standard for electromagnetic compatibility, requirements and test. However, if this equipment is operated in the presence of high levels of electromagnetic interference (EMI) or highly sensitive equipment, interference may be encountered, and the user should take whatever steps are necessary to eliminate or reduce the source of the interference. Diminished performance may lengthen operating time for the anesthetized patient. • Do not operate the Skeeter™ handpiece in the presence of Magnetic Resonance Imaging devices. • Medical Electrical Equipment needs special precautions regarding EMC and needs to be installed and put into service according to the EMC information provided in this the IPC™ or XPS™ 3000 user’s guide. • The Skeeter™ handpiece should not be used adjacent to or stacked with other equipment. If adjacent or stacked use is necessary, the Skeeter™ handpiece should be observed to verify normal operation in the configuration in which it will be used. • Portable and mobile RF communications equipment can affect Medical Electrical Equipment. • After each procedure, properly clean all reusable system components. • All service must be performed by Medtronic qualified personnel only. Repair or modification to the Skeeter™ handpiece by anyone other than qualified service personnel may significantly compromise the unit’s ability to perform effectively or void the equipment warranty. • Always inspect the components before and after use for any damage or malfunction. If damage or malfunction is observed, do not use damaged part until it is repaired or replaced. Use of damaged or malfunctioning parts can increase risk of injury. • Motors may fail due to extended use and allow a component to detach and fall from the motor causing patient injury. • Do not use an overheated device as it may cause thermal injury. Smoke or excessive heat may be caused by: • Applying excessive force while cutting (e.g., side loading) • Long operating periods (exceeding handpiece duty cycle) • Component failure or wear • The handpiece will not run properly unless the tool is in the locked or secured position. • Do not change accessory with handpiece running to prevent laceration of user and crosscontamination through compromised glove. • Do not place motor or tool on the patient or in an unsecured location during surgery. • Do not activate foot pedal without confirming the safe position and handling of the handpiece. Accidental handpiece activation could result in unintended tissue, bone, or nerve resection. • Verify reusable device was cleaned and sterilized prior to use. If not sterilized, do not use. • For warnings and precautions related to reprocessing, refer to the reprocessing instructions. • This system requires insulated connectors for the Skeeter™ handpiece. • Tools are available for resection of soft tissue and bone for surgical procedures. Use of tools depends on the intended application and patient needs. Sharp cutting powered tools induce bleeding and removal of significant tissue and bone. • Operate the tool only after the appropriate anatomical landmarks and the intended surgical site have been confirmed. Ensure adequate visualization when using powered accessories. Discontinue powered application in the event of lack of visualization of the surgical site. • Always keep the cutting area of the tool away from fingers and loose clothing to prevent laceration of user and cross-contamination through compromised glove. • Excessive noise from the tool when drilling close to the cochlea or ossicular chain may cause hearing damage. • Do not use excessive force to pry or push bone with the tool or blade during dissection. • Do not modify any system components or accessories. Use of modified components or accessories may increase risk of injury or diminish performance of the system. • Test tools for excessive vibration at desired speed before use. Vibration may be caused by eccentricity of the tool or worn components. Replace tool or handpiece, or change handpiece speed. • Test for wobble at desired speed prior to use. Discontinue use of tool if tip begins to wobble and replace tool to prevent unintended tissue removal from patient. Precautions Do not kink cables. Inspect cables and pins for cracks, tears, or corrosion. Residual risks Residual risks related to the use of the Skeeter™ handpiece include bleeding/blood loss, bone damage, fracture, nerve damage, and tissue damage/tissue trauma.
2
EN
Possible adverse events In the event that a serious incident has occurred related to device use, immediately report the event to Medtronic and the competent authorities. Materials composition This device contains nickel that may cause allergic reaction. Cobalt (CAS No. 7440-48-4) is classified as a carcinogenic, mutagenic, or toxic for reproduction (CMR) substance of Category 1A or 1B, or as an endocrine disruptor (ED), and is present at a concentration >0.1% weight/weight. For materials of concern information such as REACH, CA Prop 65, or other product stewardship programs, go to www.medtronic.com/productstewardship. Sterility The Skeeter™ handpiece is provided NON-STERILE and is intended to be sterilized before use. Follow the Reprocessing instructions to sterilize the device. Setup and use with IPC™ console Refer to figures 1, 2, 3, and 4. Skeeter™ handpiece assembly 1. Press the bur release button (Figure 2). 2. Load the desired bur for the procedure into the Skeeter™ handpiece by inserting the bur shaft through the distal end of the handpiece with a slight twisting motion while simultaneously pressing the bur release button. 3. The bur is locked into place when a “click” is noted. Locking of the bur should be checked prior to use by firmly pulling on the bur after the “click” is noted. 4. Tug the bur to ensure it fits securely in the Skeeter™ handpiece. 5. To remove the bur from the Skeeter™ handpiece, press the bur release button on the Skeeter™ handpiece and pull the bur out. Connect Skeeter™ handpiece to IPC™ console On the IPC™ console, locate the Skeeter™ handpiece accessory connection port on the connector panel (Figure 3), then insert the connector. The Skeeter™ handpiece does not use irrigation. By default, the system sets both pumps to None. Control the operation of the Skeeter™ handpiece with the IPC™ console touchscreen and the multifunction foot pedal. IPC™ console compatibility statement This handpiece has been shipped with a BF compatible connector boot (Figure 4). This connector boot is required to ensure the handpiece is compatible with the IPC™ console, which will bear a BF electrical safety rating. The IPC™ console and its accessories improves the functionality of the handpiece in several ways, including providing more torque for the Skeeter™ handpiece. The connector boot will not interfere with the ability to use the Skeeter™ handpiece with any existing Medtronic console. For questions about the connector boot or the IPC™ console, please contact your local distributor. Storage There are no special storage conditions for this device. Storage for this device should be at room temperature in a dry place. Disposal After use, dispose of this device in accordance with hospital, administrative, or government policies. Reprocessing instructions WARNINGS
• • •
This product is provided non-sterile and must be cleaned and sterilized before the first use and any reuse. Do not fully immerse, or ultrasonically clean this instrument. Do not use any cleaning instruments in the cannulated shaft of the handpiece.
Limitations on processing
Inspect the handpiece before and after reprocessing for damage and verify functionality. If damage is observed or functionality is affected, do not use the handpiece until it is repaired or replaced.
Initial treatment at the point of use
• • • •
Do not allow soil to dry on the handpiece during or after use. Remove bur from the handpiece. Disconnect the handpiece from the power source. Immediately after use, rinse the handpiece with cold to lukewarm water (10–40°C / 50–104°F) to remove all visible soil. 3
EN
Preparation before cleaning
•
Cleaning: Manual
1. Prepare a solution of a neutral enzymatic detergent at the concentration and temperature within ranges specified by the detergent manufacturer. 2. Wipe the cable and handpiece with a lint-fee cloth dipped in the prepared detergent solution. 3. Actuate the bur release button at least 3 times. 4. Scrub hard-to-clean areas (i.e. the screws, the bur release button, and any crevices) using a soft bristle brush (such as Spectrum M16). 5. Immerse the cannulated nosepiece into the prepared detergent solution up to the bur release button. While immersed, swirl gently for >20 seconds. 6. Using a syringe, inject >60 mL of the detergent solution into the distal tip of the cannulated nosepiece. 7. Immerse the cannulated nosepiece in purified water up to the bur release button. While immersed, swirl gently for >20 seconds. 8. Using a fresh syringe, inject >60 mL of purified water into the distal tip of the cannulated nosepiece. 9. Rinse the rest of the device under running purified water for >20 seconds.
Cleaning: AutoEnzymatic
1. Prepare a solution of a neutral enzymatic detergent at the concentration and temperature within ranges specified by the detergent manufacturer. 2. Immerse the cannulated nosepiece into the prepared detergent solution up to the bur release button. While immersed, swirl gently for >20 seconds. 3. Transfer the device into a washer-disinfector complying with the ISO 15883 series, orienting the instrument per washer-disinfector manufacturer’s recommendations. 4. Program and execute a cleaning cycle with the following parameters: Note: The rinse and wash times presented below are minimum validated parameters. Motor Speed: HIGH
Cycle Phase
Phase Duration
Water Type & Temperature
Detergent Type & Concentration
Pre-rinse
2 minutes
Tap or Purified Water
N/A
•
Keep the handpiece moist e.g. by covering the handpiece or its transport container with a water-soaked drape. Reprocessing should be performed as soon as is practical following use.
15–40°C (59–104°F) Enzymatic wash
2 minutes
Tap or Purified Water Detergent manufacturerrecommended temperature
Neutral wash
2 minutes
Tap or Purified Water 66–95°C (151°F–203°F) or upper label temperature limit
Thermal rinse
1 minute
Tap or Purified Water
pH-neutral enzymatic at manufacturerrecommended concentration pH-neutral non-enzymatic at manufacturerrecommended concentration N/A
90–95°C (194–203°F) Purified water
10 seconds
Purified Water 66–95°C (151–203°F)
4
N/A
EN
Cleaning: AutoAlkaline
1. Prepare a solution of a neutral enzymatic detergent at the concentration and temperature within ranges specified by the detergent manufacturer. 2. Immerse the cannulated nosepiece into the prepared detergent solution up to the bur release button. While immersed, swirl gently for >20 seconds. 3. Transfer the device into a washer-disinfector complying with the ISO 15883 series, orienting the instrument per washer-disinfector manufacturer’s recommendations. 4. Program and execute a cleaning cycle with the following parameters: Note: The rinse and wash times presented below are minimum validated parameters. Motor Speed: HIGH
Cycle Phase
Phase Duration
Water Type & Temperature
Detergent Type & Concentration
Pre-rinse
2 minutes
Tap or Purified Water
N/A
15–40°C (59–104°F) Alkaline wash
5 minutes
Tap or Purified Water
Mild-alkaline (pH 8.0–11.0) 43–95°C (110–203°F) non-enzymatic or upper label at manufacturertemperature limit recommended concentration
Rinse 1
1 minute
Tap or Purified Water
N/A
66–95°C (151–203°F) Thermal rinse
1 minute
Tap or Purified Water
N/A
90–95°C (194–203°F) Purified water
10 seconds
Purified Water
N/A
66–95°C (151–203°F) Drying
• •
Avoid water accumulation in the motor housing and in the nosepiece lumen by shaking excess water out with a downward motion. Wipe visible moisture off the handpiece using a dry, lint-free cloth.
Maintenance, inspection, and testing
Visually inspect the handpiece for presence of residual soil. Repeat cleaning if residual soil is observed.
Lubrication
• •
Packaging
•
•
Silicone spray should be sprayed into the cannulated shaft of the handpiece prior to sterilization. Apply spray until surplus lubricant is noted on the outside of the Bur Release Button. Wipe away excess lubricant from the handpiece using a clean, dry, lint-free cloth. The Skeeter™ handpiece can be sterilized alone or loaded into the Skeeter™ Sterilization Tray, P/N 3155615. The tray may additionally be loaded with a set of up to six Skeeter Oto-Flex Burs. Note: If used, the tray and burs must be cleaned before packaging per their respective, separate IFUs. Wrap the handpiece or the loaded tray using a standard sterilization wrap in accordance with industry practices (e.g. ANSI/ AAMI ST79). In the USA, an FDA-approved surgical wrap is required.
5
EN
Sterilization: Steam
Sterilize the handpiece packaged as described in the ‘Packaging’ section per one of the following steam (moist heat) sterilization cycles: Cycle Type
Gravity Displacement
Pre-Vacuum
Temperature
121°C
132°C
Exposure time
30 minutes 10 minutes 4 minutes
Drying Time*
15 minutes or until visibly dry
132°C
134°C 3 minutes
* Drying efficacy is dependent on local healthcare setting factors such as age of sterilization equipment, state of utilities etc. Medtronic recommends that sterilization wraps be inspected post steam sterilization for the presence of moisture. Recommended facility practices should be followed if moisture is observed. Sterilization: Ethylene Oxide (EO)
As an alternative to steam sterilization, sterilize the handpiece packaged as described in the ‘Packaging’ section using the following EO sterilization cycle parameters: 100% EtO Sterilization Parameters Conditioning in Chamber
Storage
54 ±2°C
Temperature
54 ±2°C
Relative Humidity
60 ±5%
Relative Humidity
60 ±5%
Vacuum Set Point
1.3 psia
Ethylene Oxide Concentration
600 ±25 mg/L
Preconditioning Time
30 minutes
Gas Exposure Time (fully cycle)
120 minutes
Aeration
8 hours heated aeration at 48–52°C or 24 hours aeration at ambient temperature
• • •
For items Contaminated with TSE Agents
Sterilization
Temperature
The handpiece must be completely dried before storage to prevent corrosion and residue deposits in the bearing and motor. Store the sterilized instrument in a clean, dry area, ensuring integrity of the sterile barrier system. Sterilized instruments whose sterile wrapping shows signs of damage or lack of integrity are considered non-sterile and must be re-sterilized before use.
Medtronic recommends incineration of devices that have directly, or indirectly, contacted patients suspected or confirmed with prions or Transmissible Spongiform Encephalopathy (TSE) such as CreutzfeldtJakob disease (CJD).
Cleaning validations were performed per AAMI TIR30. Lethality of steam sterilization cycles was validated per ISO 17665-1. Lethality of the ethylene oxide cycle was validated per ISO 11135. The instructions provided above have been validated by the manufacturer of the medical device as being capable of preparing a medical device for reuse. It remains the responsibility of the processor to ensure that the processing, as actually performed using equipment, materials and personnel in the processing facility, achieves the desired result. This requires verification and/or validation and routine monitoring of the process. Troubleshooting
6
Issue
Possible Cause
Action
Handpiece fails to rotate.
Failed handpiece motor.
Contact Customer Service.
Cables are not property connected.
Ensure handpiece cable is properly connected.
EN
Issue
Possible Cause
Action
Cables are damaged.
Check cables for cracks, splits, or bent connector pins.
Handpiece fails to operate at set speed.
Failed handpiece motor.
Contact Customer Service.
Excessive noise from the handpiece.
Failed handpiece motor.
Contact Customer Service.
Technical specifications Size
17 cm Length x 1.6 cm Diameter
Weight
57 g
Speed
1000-12000 rpm forward/reverse
Duty Cycle for Applied Part
Continuous run
Storage Temperature
-40°C to +70°C
Humidity
10% to 100% RH
Barometric Pressure
500 to 1060 hPa
Customer service For further information regarding the use of this product or to report any problems, please contact Medtronic using the appropriate information provided on the contact information card packaged with each device; or contact your local distributor. Limited warranty A.
This LIMITED WARRANTY provides assurance for the customer who purchases a Medtronic Xomed Product (hereinafter the “Product”) that should the Product fail to function to Medtronic Xomed’s published specifications during the term of this LIMITED WARRANTY (one year from the date of shipment for new Product, 90 days from date of shipment for refurbished or used Product), Medtronic Xomed will either replace, repair, or issue a credit (adjusted to reflect the age of the Product) for the Product or any portion thereof. This LIMITED WARRANTY is extended only to the buyer purchasing the Product directly from Medtronic Xomed or from its affiliate or its authorized distributor or representative.
B.
To qualify for this LIMITED WARRANTY, the following conditions must be met: (1)
The Product must be used on or before its “Use By” or “Use Before” date, if applicable.
(2)
The Product must be used in accordance with its labeling and may not be altered or subjected to misuse, abuse, accident or improper handling.
(3)
Medtronic Xomed must be notified in writing within thirty (30) days following discovery of a defect.
(4)
The Product must be returned to Medtronic Xomed within thirty (30) days of Medtronic Xomed receiving notice as provided for in (3) above.
(5)
C.
D.
Upon examination of the Product by Medtronic Xomed, Medtronic Xomed shall have determined that: (i) the Product was not repaired or altered by anyone other than Medtronic Xomed or its authorized representative, (ii) the Product was not operated under conditions other than normal use, and (iii) the prescribed periodic maintenance and services have been performed on the Product. This LIMITED WARRANTY is limited to its express terms. THIS LIMITED WARRANTY IS IN LIEU OF ALL OTHER WARRANTIES, EXPRESSED OR IMPLIED WHETHER STATUTORY OR OTHERWISE, INCLUDING ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. In no event shall Medtronic Xomed be liable for any consequential, incidental, prospective or other similar damage resulting from a defect, failure, or malfunction of the Product, whether a claim for such damage is based upon the warranty, contract, negligence or otherwise. The exclusions and limitations set out above are not intended to, and should not be construed so as to, contravene mandatory provisions of applicable law. Users may benefit from statutory warranty rights under legislation governing the sale of consumer goods. If any part or term of this LIMITED WARRANTY is held by any court of competent jurisdiction to be illegal, unenforceable, or in conflict with applicable law, the validity of the remaining portion of the LIMITED WARRANTY shall not be affected, and all rights and obligations shall be construed and enforced as if this LIMITED WARRANTY did not contain the particular part or term held to be invalid.
7
M010388C001 B 2022-04 © 2022 Medtronic, Inc.
Medtronic Xomed 6743 Southpoint Drive North Jacksonville, Florida 32216 USA +1 800 874 5797
Medtronic B.V. Earl Bakkenstraat 10 6422 PJ Heerlen The Netherlands +31 45 566 8000 Medtronic International Trading Sàrl Route du Molliau 31 Case Postale 84 CH- 1131 Tolochenaz Switzerland +41 21 802 7000
Australia Medtronic Australasia Pty Ltd 2 Alma Road Macquarie Park, NSW 2113 Australia Canada Medtronic of Canada Ltd 99 Hereford Street Brampton, Ontario L6Y 0R3 Canada +1 905 460 3800
medtronic.com manuals.medtronic.com