medtronic
StealthStation Emitter System Components Guide Rev A Oct 2021
Guide
84 Pages
Preview
Page 1
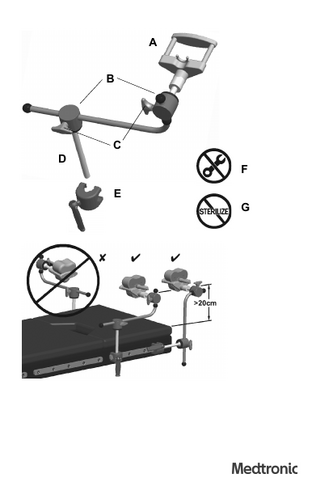
A B
C D
F E G
Emitter Holder (9733459), Emitter Holder Clamp (9733469) Components A–D. Emitter Holder (9733459) A. Cradle (9733536) B. Ball Joint C. Knob (9733537) D. Post E. Emitter Holder Clamp (9733469) F. Do not disassemble G. Do not sterilize
Intended use The Emitter Holder and Emitter Holder Clamp are intended to hold the Mobile Emitter stationary during procedures using the Medtronic Navigation Computer Assisted Surgery System.
Indications for use The StealthStation™System is intended as an aid for precisely locating anatomical structures in either open or percutaneous procedures. The StealthStation™System is indicated for any medical condition in which the use of stereotactic surgery may be appropriate, and where reference to a rigid anatomical structure such as the skull, a long bone, or vertebra can be identified relative to a CT- or MR-based model, fluoroscopy images, or digitized landmarks of the anatomy
Contraindications Medical conditions which contraindicate the use of a Medtronic computer-assisted surgery system and its associated applications include any medical conditions which may contraindicate the medical procedure itself.
Material composition Material contained in product that can cause an allergic reaction: nickel. For additional materials of concern information such as REACH, CA Prop 65, or other product stewardship programs, go to www.medtronic.com/productstewardship.
Compatibility This device is compatible with Medtronic image-guided surgery systems and Medtronic navigation instruments.
System level manuals This device can be used with Medtronic image-guided surgical systems. See the StealthStation™ system-level manuals and procedure pocket guides for descriptions of patient groups, intended users, clinical benefits, side effects, and potential complications disclosure statements. This device is an accessory to your StealthStation™ System.
Warnings Warning: Prior to use, examine the device for damage and deterioration. Do not use any compromised device. Abandon use of any device damaged during the procedure.
2
Warning: Use caution and exercise care in order to prevent injury during use. See the corresponding system-level manuals and procedure pocket guides for further handling instructions. Warning: Do not alter the product. Only use the product in accordance with its labeling. Warning: Abandon use of device if damage or malfunction occurs during a procedure. Warning: Always loosen the knobs before adjusting the emitter holder Warning: Do not reuse or reprocess the device if transmissible spongiform encephalopathies or Creutzfeldt-Jakob disease contamination is suspected. Incinerate the potentially contaminated device according to national guidelines.
Precautions Caution: The Emitter Holder and Emitter Holder Clamp are not sterile and cannot be sterilized. Sterilization may cause the device to malfunction. Caution: Properly clean the Emitter Holder and Emitter Holder Clamp prior to first use and after each use. Caution: The Emitter Holder and Emitter Holder Clamp are not intended for patient contact and should be bagged or draped if placed in sterile field. Do not adjust emitter arm joints when tightened as this may cause unexpected patient contact with non-biocompatible material. Caution: Do not disassemble the Emitter Holder or the Emitter Holder Clamp. Caution: Do not use the Emitter Holder or the Emitter Holder Clamp if any components appear to be bent or otherwise damaged. Caution: Refer to applicable software pocket guide and system level manuals for additional instructions, warnings, and cautions.
Setup and use Exact placement of the holder depends on many factors including the type of table, procedure, patient location, and patient size. Use a configuration that suits your situation. For best results follow these practices. 1. Clean the Emitter Holder and Emitter Holder Clamp before each use. 2. Attach the Emitter Holder to the OR table or the system cart by sliding the Emitter Holder Clamp over the end of the mounting rail. 3. Insert the holder post into the clamp and tighten the handle. 4. Loosen the two ball joint tightening knobs and position the holder. a. Orient the ball joints as far away from the Mobile Emitter’s electromagnetic field as possible. b. Position the holder so that it is at least 20 cm above the OR table and points toward the trackers. c. Make sure that no part of the holder extends into the Mobile Emitter’s electromagnetic field. 5. Tighten the two ball joint knobs. Warning: Always loosen the knobs before adjusting the Emitter Holder. 6. Place the Mobile Emitter in the cradle and run the cord through the notch in the cradle.
Cleaning the emitter holder and emitter holder clamp 1. Wipe and clean the exterior surfaces of the emitter holder and emitter holder clamp with a moistened low-level disinfectant wipe per the manufacturer’s instructions. Caution: Damage may result if the device is cleaned with sodium hypochlorite (bleach). 2. If disinfection is desired, make sure that the surface to be disinfected remains in contact with the disinfectant for the specified contact time as directed by the manufacturer. • The contact time refers to the minimum amount of time the surface needs to remain visibly wet.
3
• Use additional wipes, if needed, to maintain continuous contact time with the disinfectant. 3. Examine the surfaces for visible soil. If soil is present, repeat cleaning. 4. Wipe the external surfaces with a clean wipe moistened with water or 70% isopropyl alcohol to remove disinfectant residue. 1: Table 1: Compatible cleaner and disinfectant chemistries Disinfectant type Quaternary ammonium germicidal detergent solution
Disinfectant level Low level
Storage Ensure devices and packaging are dry before storing. Store in dry, clean conditions at ambient room temperature.
Disposal When the device shows visible signs of wear, it is at the end of its useful life. Dispose of it in accordance with your facility’s procedure for medical devices and national regulations.
Reporting If a serious incident occurs in relation to the use of this device, report it to Medtronic Navigation. If a serious incident occurs in the European Union, also report it to the competent authority in the Member State where the incident occurred.
Assistance If you have questions about the Emitter Holder or the Emitter Holder Clamp, contact your local Medtronic-representative or call 800 595 9709 (inside the US only) or 720 890 3160 for technical support.
Symbol definitions The following symbols may appear on device packaging: Do not disassemble
Caution: Federal law (U.S.A.) restricts this device to sale by or on the order of a physician Medical device. Importer.
4
2021-10 9733551 Revision A ©2021 Medtronic Navigation, Inc. All Rights Reserved
Medtronic Navigation, Inc. 826 Coal Creek Circle Louisville, Colorado 80027 USA Main 720 890 3200 Fax 720 890 3500 www.medtronic.com Technical Support USA 1 800 595 9709 International 720 890 3160 rs.navtechsupport@medtronic.com www.meanuals.medtronic.com
Medtronic B.V. Earl Bakkenstraat 10 6422 PJ Heerlen Netherlands Telephone 31 45 566 80 00 Australia Medtronic Australasia Pty Ltd 2 Alma Road Macquarie Park, NSW 2113 Australia Telephone 1800 668 670 Printed in the USA