medtronic
Medtronic Navigation Computer Assisted Surgery Systems
StealthStation Radiolucent Articulating Arm Instructions Rev 1 June 2020
Instructions
232 Pages
Preview
Page 1
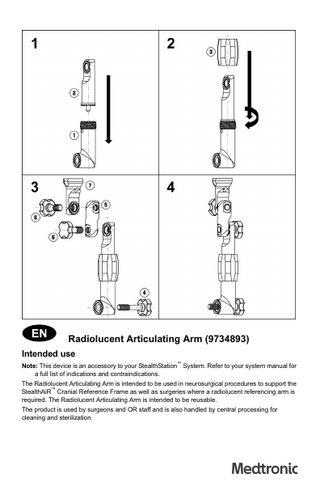
Radiolucent Articulating Arm (9734893) Intended use Note: This device is an accessory to your StealthStation™ System. Refer to your system manual for a full list of indications and contraindications. The Radiolucent Articulating Arm is intended to be used in neurosurgical procedures to support the StealthAiR™ Cranial Reference Frame as well as surgeries where a radiolucent referencing arm is required. The Radiolucent Articulating Arm is intended to be reusable. The product is used by surgeons and OR staff and is also handled by central processing for cleaning and sterilization.
Product Description The Radiolucent Articulating Arm is a reusable device that enables the Medtronic Navigation StealthAiR™ Cranial Reference Frame (9733570) to be mounted to a radiolucent headholder via a non-supplied adapter. This arm is radiolucent and MR safe, which allows for CT and MR scans. The Radiolucent Articulating Arm allows for multiple degrees of freedom, enabling the frame to be appropriately positioned toward the camera. The Radiolucent Articulating Arm can be easily disassembled for cleaning and sterilization.
Indications for use The StealthStation™ System is intended as an aid for precisely locating anatomical structures in either open or percutaneous procedures. The StealthStation™ System is indicated for any medical condition in which the use of stereotactic surgery may be appropriate, and where reference to a rigid anatomical structure such as the skull, a long bone, or vertebra can be identified relative to a CT or MR based model, fluoroscopy images, or digitized landmarks of the anatomy.
Contraindications None.
Compatibility The Radiolucent Articulating Arm is compatible with Medtronic image-guided surgery systems and Medtronic navigation instruments.
System level manuals This device can be used with Medtronic image-guided surgical systems. See the StealthStation™ system level manuals and cranial procedure Pocket Guides for descriptions of patient groups, intended users, potential clinical benefits, side effects, and potential complications. These devices are accessories to your StealthStation™ system.
Warnings Warning: Before use, examine the device for damage and deterioration. Do not use any compromised device. Abandon use of any device damaged during the procedure. Warning: During navigation, visually confirm navigational accuracy frequently by localizing on known points. Warning: The device is designed for reuse and is supplied non-sterile. Clean and sterilize it before every use according to the instructions in this document. Warning: To avoid potential exposure to blood-borne pathogens and chemicals, use appropriate Personal Protective Equipment when handling, processing, or disposing of Medtronic devices. Warning: Use caution and exercise care in order to prevent injury during use. See the corresponding system level manuals and procedure pocket guides for further handling instructions. Warning: Do not re-use or re-process a device where transmissible spongiform encephalopathy agents or Creutzfeldt-Jakob disease contamination is suspected or confirmed. Incinerate the potentially contaminated device according to national guidelines.
Precautions Caution: Federal law (U.S.A.) restricts this device to sale by or on the order of a physician. Caution: Refer to the StealthStation™ system level manuals and cranial procedure Pocket Guides for additional instructions, warnings, and cautions.
2
Assembling Warning: Do not open sterile-barrier packages or containers until surgical use. At time of use, inspect barrier for breach. If the sterile barrier is breached before surgical use, reprocess all devices contained in the package.
Radiolucent articulating arm assembly instructions 1. Section 1 (9734917) and section 2 (9734918) are joined at the ends, as shown. Then section 3 (9734919) is slipped over section 2 and screwed onto the threads of 1 to hold section 1 and section 2 together. Note: Section 3, also known as the rotation knob, has a top and bottom. The end marked with a small number 1 should be threaded onto Section 1. The end marked with a small number 2 should be threaded onto Section 2. 2. Install Screw 4 (9734922) into Section 1, as shown. 3. Attach Section 5 (9734920) to Section 2, as shown, and use one Screw 6 (9734923) to hold
them together. 4. Attach Section 7 (9734921) to Section 5, as shown, and use the other Screw 6 to hold them together. 5. Use Screw 4 to attach the Radiolucent Articulating Arm assembly to the headholder adapter.
Attaching and positioning At the Setup step in the Cranial software: 1. Secure the patient in the pmi DORO™ headholder (3034-00). The Radiolucent Articulating Arm is only compatible with the pmi DORO™ headholder (3034-00) and adapter (3033-65). 2. Mount the adapter to the headholder on the appropriate side. 3. Attach the base starburst (Section 1 in the illustration) of the Radiolucent Articulating Arm to a matching starburst on the patient headholder adapter. Tighten the base attachment screw (Screw 4) to secure the arm in place. 4. Support the arm with one hand while turning the knob or thumbscrews counterclockwise to loosen them one at a time. Note: Loosening each knob releases tension on the associated arm joint. Fully support arm segments whenever you loosen the knobs. 5. Adjust the arm linkage segments into the desired position and then retighten by hand. Do not overtighten the knob or thumbscrews or use levers, mallets, wrenches, or other tools to increase tightening torque. Warning: Slippage of the arm after registration may result in inaccurate navigation. Handtighten all connections securely. Confirm that the connections are sufficiently tightened by applying pressure against the Radiolucent Articulating Arm and verifying that it does not deflect from its locked position.
Using the Radiolucent Articulating Arm with intraoperative CT Attaching the patient reference frame 1. Select a patient reference frame. The Medtronic Navigation cranial reference StealthAiR™ Cranial Reference Frame (9733570) is a radiolucent frame that can be used with this Radiolucent Articulating Arm. Note: The bore diameter of the imaging system used with this arm and a frame must be a minimum of 40 cm. Note: When the Radiolucent Articulating Arm is used with a CT-imaging system, the minimum field of view must be 30 cm. 2. Place single-use sterile spheres on each of the tracking array stems on the reference frame. a. Push each sphere onto the stem until it snaps into place.
3
b. Make sure the sphere is firmly seated on the stem. 3. Attach the reference frame to the Radiolucent Articulating Arm by following these steps. a. Match the mounting screw and the alignment post on the underside of the patient reference frame with the corresponding screw hole and alignment notch on the top of the arm. Note: The frame can only be installed in the correct orientation. b. Hand-tighten the frame to the arm using the thumbscrew on top of the frame. c. Check to make sure that there is no movement in the frame-to-arm connection. If there is any movement in this connection, registration accuracy may be compromised. Retighten the thumbscrew until no movement is apparent. 4. Position the frame into the preferred position by loosening thumbscrews in the Radiolucent Articulating Arm, moving the frame into the desired location, and tightening the thumbscrews in place. 5. Check and hand-tighten all linkages in the Radiolucent Articulating Arm to make sure that the arm and frame are rigid and do not move in relationship to the patient. Warning: If the articulating arm is supporting a patient reference frame, and you suspect any deflection or slippage of the articulating arm after registering the patient, re-register the patient before continuing.
Preparing the Radiolucent Articulating Arm for intraoperative CT and navigation 1. Following the workflow and instructions contained in the Cranial software, perform a StealthAiR™ registration and verify registration accuracy. 2. In the Cranial software, proceed to the Navigate task. 3. Move the patient and table away from the imaging device. 4. Remove the non-sterile reference frame from the Radiolucent Articulating Arm. 5. Drape the patient and the Radiolucent Articulating Arm. Warning: Make sure that the drape does not pull down on the articulating arm, potentially displacing the mounting point from its original position. 6. Use a sterile patient reference frame to puncture the plastic drape with the mounting screw or use a sharp instrument to make a small slit in the drape and then secure the reference frame to the Radiolucent Articulating Arm mount by tightening the mounting screw through the slit in the drape. Warning: Make sure that excess drape material is not bunched in the joint between the frame and mount when tightening the mounting screw. If the drape is bunched, the sterile patient reference frame may be in a different position than the previous frame, and that difference may result in inaccurate navigation. 7. Verify registration accuracy in the Cranial software before proceeding to navigate. Warning: Slippage of the articulating arm after registration may result in inaccurate navigation. Make sure the articulating arm is locked securely in position. Do not bump or move the articulating arm. Warning: During navigation, visually confirm navigational accuracy frequently by localizing on known points. 8. After surgery, remove the patient reference frame from the Radiolucent Articulating Arm, disassemble the arm, and clean and sterilize the arm according to the instructions in this document. Clean and sterilize the patient reference frame according to the instructions provided with the frame.
Using the Radiolucent Articulating Arm with intraoperative MRI Attaching the patient reference frame Warning: The Cranial Reference Frames (961-337 and 9733881) and the StealthAiR™ Cranial Reference Frame (9733570) are MR unsafe. Remove the Cranial Reference Frame or the StealthAiR™ Cranial Reference Frame for all MR image acquisition. 1. Select a patient reference frame.
4
2. Place single-use sterile spheres on each of the tracking array stems on the reference frame. a. Push each sphere onto the stem until it snaps into place. b. Make sure the sphere is firmly seated on the stem. 3. Attach the reference frame to the Radiolucent Articulating Arm by following these steps. a. Match the mounting screw and the alignment post on the underside of the patient reference frame with the corresponding screw hole and alignment notch on the top of the arm. Note: The frame can only be installed in the correct orientation. b. Hand-tighten the frame to the arm using the thumbscrew on top of the frame. c. Check to make sure that there is no movement in the frame-to-arm connection. If there is any movement in this connection, registration accuracy may be compromised. Retighten the thumbscrew until no movement is apparent. 4. Position the frame into the preferred position by loosening thumbscrews in the Radiolucent Articulating Arm, moving the frame into the desired location, and tightening the thumbscrews in place. 5. Check and hand-tighten all linkages in the Radiolucent Articulating Arm to ensure the arm and frame are rigid and do not move in relationship to the patient. Warning: If the articulating arm is supporting a patient reference frame, and you suspect any deflection or slippage of the articulating arm after registering the patient, re-register the patient before continuing.
Preparing the Radiolucent Articulating Arm for intraoperative MRI and navigation 1. Verify that the overall patient envelope, including the patient head holder, MR coil, and Radiolucent Articulating Arm, will fit inside the iMR system magnet bore. 2. Securely attach a cranial patient reference frame to the Radiolucent Articulating Arm (see “Attaching the patient reference frame” on page 4). 3. Following the workflow and instructions contained in the Cranial software, perform a non-sterile registration using a standard technique and verify registration accuracy. 4. Remove the non-sterile reference frame from the Radiolucent Articulating Arm. 5. Drape the patient and the Radiolucent Articulating Arm. Warning: Make sure that the drape does not pull down on the articulating arm, potentially displacing the mounting point from its original position. 6. Use a sterile patient reference frame to puncture the plastic drape with the mounting screw or use a sharp instrument to make a small slit in the drape and then secure the reference frame to the Radiolucent Articulating Arm mount by tightening the mounting screw through the slit in the drape. Warning: Make sure that excess drape material is not bunched in the joint between the frame and mount when tightening the mounting screw. If the drape is bunched, the sterile patient reference frame may be in a different position than the previous frame, and that difference may result in inaccurate navigation. Note: The patient reference frame can be cleaned and then sterilized via autoclave at the time of disconnection, or you may use a second, pre-sterilized patient reference frame. After each additional intraoperative scan, replace the reference frame with a new sterile frame. 7. Verify registration accuracy in the Cranial software. Warning: Slippage of the articulating arm after registration may result in inaccurate navigation. Make sure the articulating arm is locked securely in position. Do not bump or move the articulating arm. Warning: During navigation, visually confirm navigational accuracy frequently by localizing on known points. 8. To obtain intraoperative images, follow all magnetic resonance safety procedures to make sure that the Medtronic computer-assisted surgery system and related components, including the MR-unsafe patient reference frame, are stowed properly for image acquisition. 9. Reattach a sterile patient reference frame, verify navigation accuracy, and proceed to navigate.
5
10.After surgery, remove the patient reference frame from the Radiolucent Articulating Arm, disassemble the arm, and clean and sterilize the arm according to the instructions in this document. Clean and sterilize the patient reference frame according to the instructions provided with the frame.
Cleaning, inspection, and sterilization Warning: The recommended sterilization parameters are only valid with CE-marked equipment that is properly maintained and calibrated. Caution: Because of the variability in cleaning efficiencies and sterilizer operating parameters, all given parameters (temperature, time, etc.) should be validated by persons who have training and expertise in sterilization processes. Deviation from the recommended sterilization processes is at the risk of the user facility. Limitations on Reprocessing
End of useful life is normally determined by wear and damage due to use. See the “Maintenance Inspection Testing” section in this document to determine if the device is at the end of its useful life. Caution: At the end of its useful life, dispose of the device in accordance with national regulations.
Instructions Point of Use
Do not allow blood, debris or bodily fluids to dry on the device. Remove excess soil using running, cold tap water.
Containment and Transportation
Caution: Devices should be cleaned within 30 minutes of use to limit fixation of contaminants. If the device cannot be reprocessed immediately, keep the device moist during transport.Tap water is defined as utility water with a hardness of < 150 mg/l. To prolong the life of the device, reprocess immediately after use.
Preparation for Cleaning
6
Disengage all components. Before the cleaning step, thoroughly rinse the device under running cold tap water at a temperature of 10-22°C (50-72°F) to remove any visible soil. Use a soft-bristled brush or clean cloth to aid soil removal. Give particular attention to crevices and other areas that present a challenge to cleaning. Carefully inspect devices, including any lumens and cavities, to ensure all visible soil is removed.
Automated Cleaning
Transfer the devices to the washer and select the instrument cycle. Ensure the following set of cycle parameters are properly programmed. Caution: Follow BS EN ISO 15883-1 for load and thermometric settings for all washer/disinfector machines. Note: Cycle validated using Steris Prolystica™ 2x Concentrate Enzymatic Cleaner and Steris Prolystica™ 2x Concentrate Neutral Detergent at a concentration of 1.0ml/L (1/8 oz/gal). Phase
Recirculation/Soak (minutes)
Water Temperature
Detergent Type
Prewash 1
02:00
Cold tap water N/A 10-16°C (50-61°F)
Enzyme Wash 02:00
Hot tap water 43-55°C
Neutral pH enzymatic cleaner.
(109-131°F) Wash 1
02:00
66°C (151°F) (setpoint)
Neutral pH detergent
Rinse 1
00:15
Hot tap water
N/A
43-60°C (109-140°F) Thermal Rinse
01:00
90°C (194°F) (setpoint)
N/A
Purified Water 00:10 Rinse
66°C (151°F) (setpoint)
N/A
Warning: After cleaning, visually examine all parts of the device for cleanliness. If visible soil remains, repeat cleaning.
7
Manual Cleaning
Manually clean the device only when a washer-disinfector is not available. 1. Thoroughly rinse device under running cold tap water at a temperature of 10-22˚C (50-72°F) to remove visible soil. A soft bristled brush should also be used to aid in the removal of soil. Pay particular attention to crevices, lumens and other hard-to-clean areas to remove all visible soil 2. Prepare enzymatic cleaner following the manufacturer’s recommendations of 1.0 mL/L (1/8 oz/gal) at a temperature of 23-30˚C (73-86°F). Immerse the device in the prepared enzymatic cleaner, actuate the device and allow it to soak for a minimum of 10 minutes. 3. Pay particular attention to crevices, lumens, and other hard-to-clean areas to remove all visible soil. 4. Prepare fresh enzymatic cleaner following the manufacturer’s recommendations of 1.0 mL/L (1/8 oz/gallon) at a temperature of 23-30˚C (73-86°F). Using a syringe repeatedly flush lumens, blind holes, and cracks until the solution runs clear. Allow detergent solution into hard-to-reach areas. 5. After soaking, the device should be thoroughly rinsed under running warm tap water at a temperature of 30-45˚C (86-113°F) for a minimum of 1 minute to remove all residual detergent. Actuate the device during rinse to allow water into hard-to-reach areas. Use a syringe to aid in rinsing. 6. Prepare enzymatic cleaner following the manufacturer’s recommendations of 1.0 mL/L(1/8 oz/gallon) at a temperature of 23-30˚C (73-86°F) in a sonication unit. Completely submerge the device in the prepared enzymatic cleaner and allow it to sonicate for a minimum of 10 minutes. 7. After sonication, thoroughly rinse the device under running warm tap water at a temperature of 30-45˚C (86-113°F) for a minimum of 1 minute to remove all residual cleaner. Actuate the device during rinse to allow water into hard-to-reach areas. Use a syringe to aid in rinsing. 8. Following the warm tap water rinse, thoroughly rinse the device in room temperature 23-30˚C (73-86°F) de-ionized (DI) water for a minimum of 30 seconds. Use a syringe to aid in rinsing. Warning: After cleaning, visually examine all parts of the device for cleanliness. If visible soil remains, repeat cleaning.
Drying
8
If necessary, dry the device with a clean, lint-free towel.
Maintenance Inspection Testing
Visually inspect the device before each use for obvious damage or corrosion to ensure it is not pitted, fractured, bent, loose, or otherwise damaged. Make sure of the following: • Laser etchings, engravings, and other markings are legible. • No cracks are present in any part of the device. • Discoloration, corrosion, stains or rust are not present. If so, attempt to clean using the cleaning instructions provided in this document. If reprocessing does not remove the corrosion, stains, or rust, the device is at the end of its useful life. • That the handle-to-shaft connection is secure. • There is no damage to the working ends or tips. The working end should be free from cracks, sharp-edged gouges, and other damage. • There is no damage to the threads. • All parts are present and free of damage and deterioration. • Mating ends are free of damage (nicks, gouges, bends, etc.) that would interfere with the mating function. A device that shows or exhibits properties listed above is at the end of its useful life. Dispose of the device according to national regulations. Warning: Do not reprocess for surgical use a device that has obvious damage or corrosion.
Packaging
Sterilize this device in a sterilization container with SCF01 paper filters. Caution: The device sterilization tray is to be used only for sterilization, not for cleaning.
9
Sterilization
Caution: Devices cannot be sterilized to an adequate Sterility Assurance Level without prior cleaning. Use a prevacuum steam sterilization cycle with these parameters to sterilize the device. Sterilization cycle parameters for medical facilities inside the U.S.A. • Temperature: 132°C (270°F) • Exposure Time: 4 minutes (04:00) • Minimum Dry Time: 30 minutes (30:00) Sterilization cycle parameters for medical facilities outside the U.S.A. Option A • Exposure: Prevacuum • Temperature: 132°C (270°F) • Exposure Time: 4 minutes (04:00) • Minimum Dry Time*: 30 minutes (30:00) Option B • Exposure: Prevacuum • Temperature: 134°C (273°F) • Exposure Time: 18 minutes (18:00) • Minimum Dry Time*: 30 minutes (30:00) * The time required to dry the device may vary with environmental conditions. Note: Steam for sterilization should be generated from water that has been treated to remove total dissolved solids, filtered to remove contaminants and water droplets, and supplied via piping without deadlegs or other stagnant zones where contamination might collect. Steam saturation should be greater than 97%.
Storage
Ensure instruments and sterile packaging are dry before storing. Store in dry, clean conditions at ambient room temperature such that the package is not compromised.
Disposal Dispose of contaminated devices according to your facility's biohazard disposal procedure and local and national regulations.
Contact Information If a serious incident occurs in relation to the use of these devices, report it to Medtronic Navigation. If this incident occurs in the European Union, also report it to the competent authority in the Member State where the incident occurred.
Assistance If you have questions about this device, contact your local Medtronic Navigation representative or call technical support at 1 800 595 9709 or 1 720 890 3160.
10
2020-06 9734992 G02 Revision 1 ©2020 Medtronic Navigation, Inc. All Rights Reserved
Medtronic Navigation, Inc. 826 Coal Creek Circle Louisville, Colorado 80027 USA Main 720 890 3200 Fax 720 890 3500 www.medtronic.com Technical Support USA 1 800 595 9709 International 1 720 890 3160 rs.navtechsupport@medtronic.com
Medtronic B.V. Earl Bakkenstraat 10 6422 PJ Heerlen Netherlands Telephone 31 45 566 80 00 Australia Medtronic Australasia Pty Ltd 2 Alma Road Macquarie Park, NSW 2113 1 800 668 670 Printed in the USA
ȤŋȦƮƛŭƮůŻƩŋȯʼnĖĉĚõǟȥ