medtronic
Medtronic Navigation Computer Assisted Surgery Systems
ZiehmRFD 3D Interface Instructions Rev B
Instructions
272 Pages
Preview
Page 1
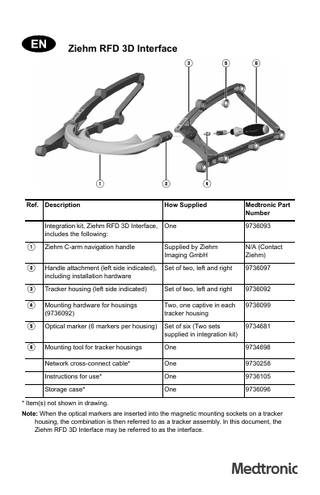
Ziehm RFD 3D Interface
Ref. Description
How Supplied
Medtronic Part Number
Integration kit, Ziehm RFD 3D Interface, includes the following:
One
9736093
1
Ziehm C-arm navigation handle
Supplied by Ziehm Imaging GmbH
N/A (Contact Ziehm)
2
Handle attachment (left side indicated), including installation hardware
Set of two, left and right
9736097
3
Tracker housing (left side indicated)
Set of two, left and right
9736092
4
Mounting hardware for housings (9736092)
Two, one captive in each tracker housing
9736099
5
Optical marker (6 markers per housing)
Set of six (Two sets supplied in integration kit)
9734681
6
Mounting tool for tracker housings
One
9734698
Network cross-connect cable*
One
9730258
Instructions for use*
One
9736105
Storage case*
One
9736096
* Item(s) not shown in drawing. Note: When the optical markers are inserted into the magnetic mounting sockets on a tracker housing, the combination is then referred to as a tracker assembly. In this document, the Ziehm RFD 3D Interface may be referred to as the interface.
Ziehm RFD 3D Interface
Intended use The Ziehm RFD 3D Interface is an accessory to the StealthStation™ system. The Ziehm RFD 3D Interface is intended to enable images of the patient’s anatomy that have been acquired from the Ziehm RFD 3D C-arm to be used with the Medtronic computer-assisted surgery system.
Indications for use The StealthStation™ System is intended as an aid for precisely locating anatomical structures in either open or percutaneous surgical procedures. The StealthStation™ System is indicated for any medical condition in which the use of stereotactic surgery may be appropriate, and where reference to a rigid anatomical structure such as the skull, a long bone, or vertebra can be identified relative to a CT- or MRbased model, fluoroscopy images, or digitized landmarks of the anatomy.
Contraindications None
Material composition Material contained in product that can cause an allergic reaction: nickel
Operating principle The Ziehm RFD 3D Interface enables optical tracking of the C-arm detector panel by the StealthStation™ camera. The camera uses the position of optical markers on the tracker assembly and the position of the patient reference frame to determine the relationship between the image volume and the patient anatomy. After acquiring an image, the image is transferred from Ziehm RFD 3D to the StealthStation™, and it is registered automatically in the StealthStation™ clinical software for use in navigation.
Compatible systems The Ziehm RFD 3D Interface is compatible with Ziehm Vision RFD 3D C-arms with Ziehm 3DApp software version 1.07.0 or later and with StealthStation™ S8 systems with Spine software version 1.1.0, 1.2.0, 2.0.0, or later. The Ziehm RFD 3D Interface is not compatible with StealthStation™ S7 or other legacy Medtronic systems. Note: Features described for the interface apply only to 3D scans and not to 2D scans.
System level manuals This device can be used with Medtronic image-guided surgical systems. See the StealthStation™ system level manuals and pocket guides for descriptions of patient groups, intended users, potential clinical benefits, side effects, and potential complications. These devices are accessories to your StealthStation™ System.
2
Ziehm RFD 3D Interface
Warnings Warning: The Ziehm RFD 3D Interface should be used only by qualified medical professionals who are trained in performing surgery and are familiar with computer-assisted surgery systems. Warning: Use care to prevent injury while handling the tracker assembly. Warning: Do not modify any of the interface components. Warning: Do not disassemble any of the interface components. Warning: Use caution and exercise care in order to prevent injury during use. See the corresponding system level manuals and procedure pocket guides for further handling instructions. Warning: Prior to use, examine the device. If you find any damage or deterioration, do not use the device and do not attempt to repair it. Warning: Abandon use if damage or malfunction occurs during a procedure. Warning: To avoid potential exposure to blood-borne pathogens and chemicals, use appropriate Personal Protective Equipment when handling, processing, or disposing of Medtronic devices.
Precautions Caution: Use care to prevent deformation of the tracker assembly during handling. Caution: Clean optical markers before each use. Caution: The Ziehm RFD 3D Interface contains no user-serviceable parts. Caution: Do not sterilize any components of the Ziehm RFD 3D Interface. The high temperatures of sterilization may damage the parts. Caution: Federal law (U.S.A.) restricts this device to sale by or on the order of a physician. Caution: During navigation with the Ziehm RFD 3D Interface, visually confirm navigational accuracy frequently by localizing on known points. Caution: Refer to the StealthStation™ system level manual and procedure pocket guides for additional instructions, warnings, and cautions.
Preparing to use the interface Mounting a tracker housing to the C-arm Note: It is not necessary to mount both tracker assemblies unless you need both for the procedure. Handle attachments must be installed on and calibrated to the C-arm only by Medtronic field service personnel. After successful calibration, users may mount and unmount either or both tracker housings as needed. Re-calibration is only necessary if the Ziehm C-arm navigation handle or a handle attachment has been tampered with, when damage to any part of the interface is
3
Ziehm RFD 3D Interface
suspected, or as part of periodic maintenance, including updates or service to the Carm by Ziehm Imaging GmbH. Inspect the tracker assemblies and handle attachments for damage before use. Inspect the tracker assemblies and handle attachments for cleanliness before use. Warning: Do not use the tracker assembly or handle attachment if they are not clean. Warning: Use a tracker assembly only with the handle attachment(s) and the Carm(s) with which it was calibrated. Warning: Do not use a tracker assembly or handle attachment if either is damaged. Warning: If mishandling has been observed or is suspected, the tracker assembly and handle attachment must be re-calibrated by Medtronic field service personnel. Warning: Never mount or unmount a tracker housing while the equipment is positioned over a patient. Warning: Follow the C-arm manufacturer’s instructions for use. To mount a tracker housing: 1. Rotate the C-arm to a comfortable working position and secure the C-arm in place before mounting either tracker housing. 2. Position the left or right tracker housing over its respective handle attachment. 3. Use the mounting tool to tighten the mounting screw through the tracker housing and into the handle attachment. Tighten clockwise until the tool clicks. 4. Repeat the steps above for the other tracker housing, if needed.
Inserting optical markers Keep extra optical markers on site. The markers are available in replacement packs of six markers (9734681). Over time, markers can become scratched or otherwise occluded, leading to optical failure. Inspect markers during insertion; replace any markers suspected of being faulty. Warning: Failure to follow the optical marker insertion instructions may result in navigational inaccuracy. Caution: Clean optical markers before each use. Do not use any sodium hypochlorite solution (bleach) on markers because it can damage the markers. 1. Before inserting an optical marker into a mounting socket, wipe out the socket with a lint-free cloth to ensure that no debris impedes the fit of the marker into the socket. 2. Align an optical marker over the empty mounting socket until you feel magnetic force pulling on the marker. 3. Release the marker and allow it to snap into the mounting socket. 4. Test that the marker is fully seated in its magnetic socket by rotating the marker within the socket. It should rotate freely.
4
Ziehm RFD 3D Interface
5. Visually inspect the optical marker to make sure that it is fully seated within the mounting socket, and for cleanliness.
Draping the detector with the tracker assembly in place To drape the Ziehm RFD 3D C-arm detector, as well as any mounted tracker assembly, use the appropriate cover set from Ziehm Imaging GmbH. 1. Inspect the tracker assembly for damage and cleanliness. 2. Orient the drape so that the straight-edged, non-elastic portion aligns with the tracker assembly. Make sure that the entire tracker assembly is covered without putting undue stress on the tracker assembly. 3. Smooth the drape against the optical markers. Make sure that there are no wrinkles or labels obscuring the camera’s view of the markers. Note: Maintain visibility of the LEDs by making sure that they are clearly visible and unobstructed by labels and any wrinkles in the drape.
Final steps for use Warning: Confirm image registration accuracy prior to navigation, and frequently confirm accuracy during navigation. Abort the use of the Ziehm RFD 3D Interface if navigation is inaccurate and steps to restore accuracy are unsuccessful. Note: The tracker assembly and patient reference frame must be visible to the camera in the tracking view at the beginning of a 3D scan. (If the optical markers pass partly or completely out of the camera’s view during a scan, this will not affect image acquisition.) Also refer to the procedure-specific instructions for use, such as the StealthStation™ S8 Spine with O-arm™ and 3D Fluoro Imaging Pocket Guide (9735759), for additional instructions, symbols, warnings, and cautions. 1. Power on both the Ziehm RFD 3D C-arm and the Medtronic StealthStation™ system. 2. Connect the StealthStation™ system to the C-arm with an Ethernet cable. 3. Log-in to the StealthStation™ S8 Spine software, and then select the procedure. 4. In the Equipment task, if it isn’t already selected, add the Ziehm RFD 3D Carm. Verify that the serial number of the mounted tracker housing matches the serial number in the selected procedure. 5. Move through the steps in the application to the Images task, then: a. Position the C-arm and StealthStation™ camera for image acquisition. b. Ensure that the tracker assembly and patient reference frame are tracking in the software. c. Acquire 3D fluoroscopic images with the Ziehm RFD 3D C-arm according to the protocol in the Ziehm RFD 3D User Manual. d. Verify that the patient anatomy images are correct and in the proper orientation for the procedure. 5
Ziehm RFD 3D Interface
6. Proceed with navigation.
Unmounting a tracker housing from the C-arm Carry out the following procedure before moving the C-arm between rooms or when preparing to store the interface components. Warning: Never mount or unmount a tracker housing while the equipment is positioned over a patient. Warning: Do not remove handle attachments from the Ziehm C-arm navigation handle; doing so will invalidate the calibration of the interface. Re-installation of the handle attachments and re-calibration by Medtronic field personnel will be required. Warning: Unmount, clean, and store tracker housings after use and before moving or storing the C-arm. Moving or storing the C-arm with the tracker housings mounted risks damage to the housings, and this can lead to navigation inaccuracy. 1. Rotate the C-arm to a comfortable position and secure it in place before unmounting the tracker housing from the handle attachment. 2. Use the mounting tool to loosen the mounting screw that holds the housing on the handle attachment. 3. Clean the interface components used in the procedure according to the “Cleaning the equipment” section on page 6. 4. Carefully place the tracker housing and other components in the storage case. 5. Repeat the steps above for the other tracker housing, if it was mounted.
Removing optical markers Warning: Never use a device to pry an optical marker out of its socket. The prying action can damage the marker or socket, which can result in navigation inaccuracy. Warning: Remove, clean, and store optical markers after use. Failing to store markers properly can lead to marker damage and thus to navigation inaccuracy. 1. Use your fingers to grip an optical marker in its magnetic mounting socket. 2. Pull firmly to remove the marker from its socket.
Cleaning the equipment Caution: Do not sterilize any components of the Ziehm RFD 3D Interface. The high temperatures of sterilization may damage the parts. Note: -
6
For optical markers: Remove optical markers from tracker housings before cleaning the markers. Do not immerse optical markers. To prolong marker life, use microfiber cloths instead of wipes.
Ziehm RFD 3D Interface
-
For tracker housings: Remove optical markers from tracker housings before cleaning the housings. Do not immerse tracker housings.
-
For handle attachments: Do not remove handle attachments from the Ziehm C-arm navigation handle. Clean the handle attachments in place. Table 1: Compatible cleaner and disinfectant chemistries Disinfectant type
Disinfectant level
Quaternary ammonium germicidal detergent solution
Low-level
Isopropyl alcohol (70%)
Low-level
General cleaning instructions Components of the interface which may require cleaning or disinfection include optical markers, tracker housings, and handle attachments. See the notes about those components above. 1. Use a disinfectant that is compatible with the interface component according to Table 1. 2. Wipe and clean surfaces with a moistened, low-level disinfectant wipe according to the wipe manufacturer’s instructions. If a premoistened wipe is not available, use a clean wipe and moisten it with disinfectant. Do not saturate the wipe so much that liquid can enter openings in the parts to be cleaned. 3. If disinfection is desired, make sure that the surface remains in contact with the disinfectant for the time specified by the manufacturer of the disinfectant. -
Contact time refers to the minimum time that the surface needs to remain visibly wet.
-
Use additional wipes as needed to maintain continuous contact time with the disinfectant.
4. Examine the surfaces for visible soil. If soil is present, repeat cleaning. 5. To remove disinfectant residue, wipe surfaces with a clean cloth moistened with sterile water or 70% isopropyl alcohol.
Maintenance Medtronic recommends annual periodic maintenance of the interface by Medtronic personnel. This includes re-calibration. Users may schedule their own inspection periods for replaceable items such as optical markers and mounting hardware. To order replacements, contact Medtronic Navigation technical support.
Storage The Ziehm RFD 3D Interface is shipped in a custom storage case with foam fittings designed to hold the tracker housings and related items securely. Keep these instructions inside the case for reference. Contact technical support for instructions for return shipping of the Ziehm RFD 3D Interface. 7
Ziehm RFD 3D Interface
Disposal Dispose of contaminated devices according to your facility's biohazard disposal procedure and local and national regulations.
Contact information If a serious incident occurs in relation to the use of this device, report it to Medtronic Navigation. If this incident occurs in the European Union, also report it to the competent authority in the Member State where the incident occurred.
Assistance For questions about the Ziehm RFD 3D Interface, or for return shipping information, contact your local Medtronic Navigation representative or call technical support at 1 800 595 9709 or 1 720 890 3160.
Specifications Operating conditions
Temperature: 18°C to 30°C (64°F to 86°F). Relative humidity: 80% or less, non-condensing.
Storage conditions
Temperature: -5°C to 55°C (23°F to 131°F). Relative humidity: 80% or less, non-condensing.
Shipping/Transport conditions
Temperature: -29°C to 60°C (-20°F to 140°F). Relative humidity: 80% or less, non-condensing.
Useful life
The useful life of the Ziehm RFD 3D Interface, excluding replaceable parts such as optical markers and mounting hardware, is 5 years.
Symbol definitions The following symbols may appear on devices or packaging: Caution: Federal law (U.S.A.) restricts this device to sale by or on the order of a physician Do not sterilize Do not disassemble
Use the supplied mounting tool to tighten mounting screws until the tool clicks
Medical device
Ziehm product names are the property of Ziehm Imaging GmbH.
8
2023-07 9736105 G02 Revision B ©2023 Medtronic Navigation, Inc. All Rights Reserved
Medtronic Navigation, Inc. 826 Coal Creek Circle Louisville, Colorado 80027 USA Main 720 890 3200 Fax 720 890 3500 www.medtronic.com Technical Support: USA 1 800 595 9709 International 720 890 3160 rs.navtechsupport@medtronic.com
Medtronic B.V. Earl Bakkenstraat 10 6422 PJ Heerlen Netherlands Telephone 31 45 566 80 00 Australia Medtronic Australasia Pty Ltd 2 Alma Road Macquarie Park, NSW 2113 1800 668 670 Printed in the USA
ȤŋȦƮƛŭƮŲŶŢŋȯʼnĖĉĚĆǹȥ