medtronic
Medtronic Nerve Integrity Monitors ( NIM )
NIM - Pulse 3.0 Nerve Integrity Monitor Users Guide Aug 2009
Users Guide
38 Pages
Preview
Page 1
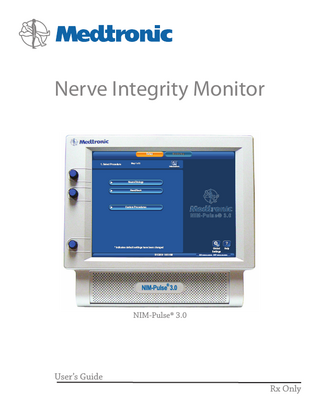
Nerve Integrity Monitor Setup
1. Select Procedure
Monitoring
Step 1 of 2 Information
Neuro/Otology Head/Neck
Custom Procedures
NIM-Pulse® 3.0
?
* indicates default settings have been changed 5/1/2009 9:00 AM
Global Settings
Help
GUI vxxxx.x.xxxxx DSP vxxx.x.xx.xxxx
®
NIM-Pulse 3.0
NIM-Pulse® 3.0
User’s Guide Rx Only
MEDTRONIC XOMED INC. 6743 Southpoint Drive North Jacksonville, FL 32216 USA
™ are trademarks and ® are registered marks of Medtronic Xomed, Inc. HP DeskJet™ is a trademark of Hewlett Packard Company © 2009 Medtronic, Inc. Released documents are available for viewing/printing @ www.medtronicENT-TechComms.com
The information contained in this document was accurate at time of publication. Medtronic reserves the right to make changes in the product described in this manual without notice and without incorporating those changes in any products already sold.
Contents Contents...3 Definitions (used in this manual)...4 Warnings and Precautions...4 Warnings...4 System Warnings...4 Precautions...4
Symbols...5 Buttons and Indicators ...6 When the System Arrives...7 Unpacking and Inspection...7 Software...7
System Description...7 Device Description...7 Indications for Use...7 Contraindications...7
Contents Patient Interface and Stimulator Combinations...19 Monopolar Probe and Stimulus Dissection Probe...19 Bipolar and Monopolar Probe ...19
Monitor Set‑up...20 Basic Set‑up All Procedures...20 Standard Set‑up...20 Custom Set‑up...20 When the Case is Complete...20 When Monitoring is Complete...20 Power Disconnection...20
Cleaning and Maintenance...21 Cleaning (after each use) ...21 Storage...21 Maintenance...21 Fuses...21 Console Replacement...21 Patient Interface Replacement...22
Customer Care...7
Troubleshooting...23 Technical Specifications ...25 Appendix A Accessories / Parts List...27
Components...7
Appendix B Annual System Quick Check...28
Medtronic Xomed, Inc. ...7 Help Line...7 International Service...7 Console Front...7 Console Left Side...7 Console Rear...8 Patient Interface...8 Patient Simulator...8 Stimulator Probes/Handles...8 Monopolar...8 Bipolar...8 Muting Detector...9 Electrodes...9 Power Cords...9 The Splash Screen...9 Self Test...9
Set‑Up Mode...9
Select Procedure Step 1 of 2...9 Global Settings...10 Help...10 Place Electrodes Step 2 of 2...10 Electrode Check...10 Electrode Check Panel...11 Electrode Check Panel Pass/Fail...11 Electrode Check Show Details Panel...11 Electrode Type Panel...11 Electrode Troubleshooting Guide...11 Case Information...12 Procedure Settings Panel...12 Advanced Settings...12 Audio Tab...12 Monitoring Tab...13 Stimulation Tab...14
System Components & Accessories...27 Preventive and Corrective Maintenance...28
Appendix C Patient Simulator Instructions for Use...29 Introduction...29 System Description...29 System Set‑Up...29 Simulator Set‑Up...29 System Assessment...29 Confirming Electrodes...29 Electrode Lead Off...30 Stimulation...30 Stimulus: Set and Measure...30 Cleaning...31 Storage...31 Troubleshooting ...31 Appendix D The NIM® Equipment Cart...32 Uncrating...32 NIM® Tether...32 Appendix E Default Tables ...33 Channel Default Settings...33 Display Default Settings...33 STIM 1 & 2 Default Settings...33 Appendix F Electromagnetic Immunity...34 Guidance and manufacturer’s declaration – electromagnetic immunity - Part I...34 Guidance and manufacturer’s declaration – electromagnetic immunity - Part II...35
Limited Warranty...36
Monitoring Mode...14 Control Panel...15
Special Functions and Features...16 Visual Alarms and Warnings...16 Audio – Understanding What You Hear...16 Alarms...16 Voice Annunciation...16 Stimulus Delivery Audio...16 Muting ...17 Muting Conditions...17 STIM Bur Guard...17
System Set‑up...17 Operating Room Set‑up...17 Typical Set-Up (shown with IPC™)...17 Anesthesia Requirements...17 Muting Detector Set‑up...17
Patient Interface Set‑up...18 Patient Interface and Single Stimulators ...18 Monopolar Probe with Universal Handle...18 Bipolar Probe...19
Nerve Integrity Monitor
3
Warnings and Precautions
Definitions (used in this manual) FCU NIM® NIM 3.0 Event Sequence Stimulus Rejection Period GUI DSP ABR SSEP
Foot Control Unit Nerve Integrity Monitor NIM-Pulse® 3.0 A sequence is defined as a series of events separated from each other by less than one second. Adjustable delay reading EMG after stimulation. In previous versions of the NIM, this was referred to as Stimulus Artifact or Artifact Delay. Graphic User Interface Digital Signal Processor Auditory Brainstem Response Somatosensory
Warnings and Precautions
It is important that the NIM-Pulse® 3.0 intended operators be familiar with this manual: its Warnings, Precautions, procedures and safety issues. Disregarding the information on safety is considered abnormal use.
Warnings System Warnings
W1. After each procedure, properly clean and disinfect all reusable system components. W2. To avoid the risk of fire or explosion, do not use the NIM® System in the presence of flammable anesthetics and/or oxygen rich environment. W3. Disconnect power to the NIM-Pulse® 3.0 Console before cleaning the unit to avoid electrical macro shock. W4. Achieve electrical grounding reliability with proper connections. Connect the NIM-Pulse® 3.0 Console to hospital grade receptacles only. W5. DO NOT use any parts other than Medtronic Xomed, Inc. components as damage or substandard performance could result. W6. This medical device complies with IEC/EN60601-1-2 safety standard for electromagnetic compatibility, requirements and test. However, if this equipment is operated in the presence of high levels of electromagnetic interference (EMI) or highly sensitive equipment, interference may be encountered and the user should take whatever steps are necessary to eliminate or reduce the source of the interference. Diminished performance may lengthen operating time for anesthetized patient. W7. It is important that the NIM-Pulse® 3.0 operator be familiar with this manual, its precautions, procedures and safety issues. W8. To avoid electrical shock, do not attach unapproved components or accessories to the NIM® System. W9. All service must be performed by Medtronic qualified personnel only. W10. To avoid patient burns: a. Do not activate the electrosurgical instruments while stimulator is in contact with tissue. b. Do not leave stimulating electrodes or probes in surgical field. c. Do not store stimulating electrodes or probes in electrosurgical instrument holder. d. Do not allow a second surgeon to use electrosurgical instruments while stimulator is in use. W11. Direct stimulator contact may disrupt the operation of active implanted devices. Consult medical specialist before use. W12. Electrocardiogram monitoring artifacts may be caused by NIM® stimulus current delivery or EMG electrode impedance monitoring. W13. Use of unapproved stimulators, stimulus probes, stimulus dissection instruments or electrodes may result in compromised NIM® operation, such as, but not limited to decreased accuracy. W14. Repair and/or modification to the NIM® or any accessory by anyone other than qualified service personnel may significantly compromise the unit’s ability to monitor nerve activity and/or void the equipment warranty. 4
W15. The NIM® does not prevent the surgical severing of nerves. If monitoring is compromised, the surgical practitioner must rely on alternate methods, or surgical skills, experience, and anatomical knowledge to prevent damage to nerves. W16. If paralyzing anesthetic agents have been used, patient must regain muscle activity prior to use of the NIM-Pulse® 3.0 EMG Monitor. W17. To avoid the risk of infection while using the NIM® Stylus, the user must maintain good sterility practices. W18. False negative responses (failure to locate nerve) may result from: a. Shorted EMG electrode or cabling (conductive parts of applied needle electrodes or cables contacting each other). b. Patient Interface fuse blown (32 mA, 250 V. Xomed Part No.: 8250615). c. Patient Interface defective. d. Inadequate stimulus current. e. Inadequate current for stimulation of nerve through hardware, such as stimulus dissection instruments, may vary based on the physical size, shape characteristics, and design of the hardware and proximity to the nerve. f. Inadvertent simultaneous current delivery from both Stimulator (Patient Interface) probe outputs. This may result in current shunting, division between the stimulator probes. g. Shorted internal amplifier (characterized by baseline activity of < 3 μV p-p). W19. Stimulator current may cause involuntary patient movement resulting in patient injury. W20. Anesthetic agents used may have an effect on the EMG amplitude. W21. Not for NIM-Pulse® 3.0. W22. EMG amplitude may be affected by anesthesia regimen used. Consult anesthesiologist if EMG changes are observed. W23. Electrode integrity should be checked after electrode insertion and before electrode removal to give additional assurance that electrode continuity was maintained throughout the entire procedure. If electrode impedance is very high, discontinue use and replace. W24. Not for NIM-Pulse® 3.0. W25. Avoid trans-thoracic stimulation; when possible, maintain anode and cathode stimulating sites in close proximity. W26. Operation in close proximity to a shortwave or microwave therapy equipment may produce instability in the electrical stimulator output. W27. Safe stimulus levels are dependent on various conditions including but not limited to: type of excitable tissue, Charge Per Pulse, and Charge Per Unit Area. Waveform morphology, repetition rate, and stimulator effective surface area must be considered. Special operator attention is required for stimulus levels which exceed default settings or conditions resulting in levels higher than 2 mA RMS/cm2.
Precautions P1. P2. P3. P4.
P5. P6.
Medical Electrical Equipment needs special precautions regarding EMC and needs to be installed and put into service according to the EMC information provided in this Guide. Portable and mobile RF communications equipment can affect Medical Electrical Equipment. Use of accessories and cables other than those specified and sold by Medtronic may result in increased emissions and decreased immunity of this unit. The NIM-Pulse® 3.0 should not be used adjacent to or stacked with other equipment. If adjacent or stacked use is necessary, the NIM-Pulse® 3.0 should be observed to verify normal operation in the configuration in which it will be used. Loud extraneous monitoring noise is caused by activation of electrosurgical unit. Muting Detector must be properly attached to the active electrosurgical lead. Inability to deliver stimulus current flow may be caused by inadvertent simultaneous current delivery from both STIM 1 probe outputs. This may result in current shunting, division between the stimulator probes.
Nerve Integrity Monitor
Symbols P7. P8.
Avoid accidental contact between ‘PATIENT APPLIED PARTS’ and other conductive parts including those connected to protective earth. The NEW Muting Probe (Ref - 8220325) is compatible with previous versions of the NIM. However, previous versions of the Muting Probe are NOT compatible with the NIM 3.0 System.
Equipotential Consult Instructions For Use
Symbols
Attention See Instructions For Use
Serial Number Do not dispose of this product in the unsorted municipal waste stream. Dispose of this product according to local regulations. See http://recycling.Medtronic.Com for instructions on proper disposal of this product. Do Not Use If Package Is Open Or Damaged. Package Contents
Use By Date Do Not Reuse Lot Number Fuse Accessory
.
Protected Against Vertical Water Drops. Protected Against The Effects Of Temporary Immersion In Water. Rated For Water Ingress (IEC 60529) Type BF Applied Part Manual Start/Stop Rf Transmitter (Interference May Occur) Snapshot Option - Open Comments Dialog Box. Snapshot Option - Send Snapshot to Printer. and Indicates a Printer is connected. Snapshot Option - Send Snapshot to USB Storage Device. and Indicates a USB Storage Device is connected.
Catalog Number AC Power Output Is Approximately Equal To Sterilized By Radiation. Do Not Use If Package Is Open Or Damaged. Non Sterile Sterilized By Ethylene Oxide. Do Not Use If Package Is Open Or Damaged. Authorized Representative In The European Community. This Device Complies With Medical Device Directive 93/42/EEC Caution: Federal Law (U.S.A.) Restricts This Device To Sale By Or On The Order Of A Physician. Quantity Manufacturer Date Of Manufacture ROHS - Environmental Friendly Use Period - China (SJ/ T11364-2006). Conforms To IEC/EN60601-1 Certified To CSA C22.2 No.601.1 Protective Earth
Nerve Integrity Monitor
5
Buttons and Indicators
Buttons and Indicators
Freeze Button: Freezes entire screen (all channels) Snapshot Button: Saves current screen to memory or to selected peripheral device.
In this section all buttons used on the “Touch Screen User Interface” are displayed with an explanation of how they work. Radio Button: Deselected For option selection where choice is limited to one of two or more options.
Activate Button: Activates STIM 2 stimulus adjustment buttons. Electrode Check Button: Opens Electrode Status Panel
Radio Button: Selected
Delete/Close Button: Closes “Delete Procedure” dialog box Opens “Delete a Custom Procedure” dialog box Global Settings Button: Global Settings allows the user to select screen language, date/ time format and the Diagnostic Mode, as well as set system date/time and Restore Factory Defaults Information Button: Opens Information Screen to enter: • Surgeon’s Name • Patient’s Name • Notes
Check Box: Deselected For option selection where choice is to enable or disable a single or multiple options. Check Box: Selected
EMG Audio and Event Tones Check Boxes: One or both must be selected. Both cannot be deselected. Red X: Indicates a failed test.
Next Button: Opens the next screen or graphic display
Green Check: Indicates a successfully passed test.
Previous Button: Opens the previous screen or graphic display
No Button: Do not Accept/Keep
Select Button: Option Button See associated text indicating option. Help Button: Opens Help Screen for Electrode Placement & Sound Samples
Measure Button: To view details of the event waveform. Advanced Settings Button Opens: Audio, Monitoring, and Stimulation Display Button: Opens panel for adjusting amplitude and time scales. Save Button: Sends current screen to USB mass storage device. Print Button: Sends current screen to printer
6
2
Channel Buttons: Channels can be turned On, Off or Muted
Decrease/Increase Buttons and Setting Display: Used to make adjustments to the subject as defined in the open panel. Setup Setup Setup
Multi State Buttons (Set‑Up used as an example): Gray = Inactive (not selectable) Blue = Selectable Orange = Selected Set‑Up Button: Opens/Starts the setup process Monitor Button: Opens the Main/Monitoring Screen
Program Loading Indicator
Accept Button: Function as indicated. Repeat Button: Function as indicated.
Increase Button: Increases value/ Setting
Monitor Button: Opens Monitoring Screen
1
Yes Button: Accept/Keep
Orange Check: Indicates an Active Channel.
Decrease Button: Decreases value/Setting
Channels Button: Opens a drop-down menu used to name channels.
Cancel Button: Function as indicated.
Ω
Show Details Button: Used to show impedance readings Hide Details Button: Used to hide impedance readings OK Button: Used to close panels Scroll Up/Down Buttons: Used to scroll through selected events Restore Button: Used to restore factory defaults. Mute Button: Used to mute channel. Unmute Button: Used to unmute channel. Nerve Integrity Monitor
When the System Arrives
Components
Unpacking and Inspection
Console Front
Check off the contents of the box against packing slip. If incomplete or damaged, notify Customer Care. If container is damaged, or cushioning material shows stress, notify carrier and Customer Care. Keep shipping materials for carrier inspection. After unpacking, save the cartons and packing material. If the instrument is to be shipped the shipping package will provide proper protection.
Components
Software
Software information (manufacturer, version, and release date) is contained on a card packaged with the system. Save this card for future reference.
System Description Device Description
The NIM-Pulse® 3.0 is a two-channel EMG monitor for intraoperative use during surgeries in which a nerve is at risk due to unintentional manipulation. The NIM 3.0 System records electromyographic (EMG) activity from muscles innervated by the affected nerve. The monitor will assist early nerve identification by providing the surgeon with a tool to help locate and identify the particular nerve at risk within the surgical field. It will continuously monitor EMG activity from the muscles innervated by the nerve at risk to minimize trauma by alerting the surgeon when a particular nerve has been activated. The monitor utilizes touch screen and color graphic user interface (GUI) along with the audio feedback to increase the usability of the device.
Indications for Use
The NIM 3.0 is intended for locating and identifying cranial and peripheral motor and mixed motor-sensory nerves during surgery, including spinal cord and spinal nerve roots. Indications for NIM 3.0 EMG Monitoring Procedures include: Intracranial, Extracranial, Intratemporal, Extratemporal, Neck Dissections, Thoracic Surgeries, and Upper and Lower Extremities Indications for Spinal procedures which may use NIM 3.0 EMG monitoring include: Degenerative Treatments, Pedicle Screw Procedures, Fusion Cages, Rhizotomy, Orthopedic Surgery, Open and Percutaneous Lumbar and Cervical Surgical Procedures, and Thoracic Surgical Procedures
A. STIM 1 stimulus adjustment. B. STIM 2 stimulus adjustment. C. Volume adjustment. D. The Speaker provides audio alarms, acoustic EMG monitoring, and voice prompts. E. Product name. F. Touchscreen – The Touch Screen displays EMG waveforms and controls many of the functions of the NIM 3.0.
Console Left Side
Contraindications
The NIM 3.0 is contraindicated for use with paralyzing anesthetic agents that will significantly reduce, if not completely eliminate, EMG responses to direct or passive nerve stimulation.
Customer Care Medtronic Xomed, Inc. 6743 Southpoint Drive North Jacksonville, FL 32216 USA www.medtronicENT.com
Help Line
(800)-874-5797
International Service
International customers should contact their local Medtronic Xomed office.
Nerve Integrity Monitor
A. USB Out: The USB Out is an industry standard USB type connector that can be used with mass storage devices. B. Anti-Glare Stand: This device is used to change the viewing angle of the NIM 3.0 screen. It is shown in the tilted (up) position.
7
Components
Console Rear
G. Positive Electrode Jacks: Positive electrodes have matching colorcoded wires and plugs. H. The Patient Interface fuses are for Stimulator Output and are specifically tested for ECU protection. Use Xomed 11270048 Fuse, 5 x20mm, 32mA, 250 V. Order 8253075 Fuse Kit for replacements. Negative Electrode Jacks: Negative electrodes have black wires and color-coded plugs.
O K
N
E
M
Patient Simulator
The Patient Simulator is used for troubleshooting and demonstrating the system without the need for patient interaction.
L
A B C
J
F
D G
H
I
P
A. Accessory Power Outlet: The Accessory Power Outlet used with the approved NIM 3.0 Accessories (i.e. the approved printer power supply only). B. Fuse Access: The AC power fuses are located on the back of the units. C. Power Switch: The power switch turns the power ON or OFF. D. Power Connector: The power cord plugs into the back of the NIM 3.0 System console. The input fuses and accessory output is in the power entry module. Plug the power cord into the A/C power outlet. E. Equipotential: Uniform potential. F. For future use. G. USB Out: The USB Out is an industry standard USB type connector (two port) that can be used with mass storage devices/printer/ keyboard. H. VGA Output: Not active on NIM-Pulse® 3.0 System. I. Surgeon Mini Screen Port: Output connection to Surgeon Mini Screen or ad. J. Muting Detector Input: Near-field radio frequency detector. K. Patient Interface Connector: The patient interface connector is a 25-pin D-sub. L. Not active on NIM-Pulse® 3.0 System. M. RCR Audio Jack: An RCA audio jack is provided to output an audio signal that can be overlaid onto a video signal when using industry standard recording devices. The output will be audio line level (1 Vp-p). N. Mini Jack: Standard configuration is for private listening through Stereo Headphones. O. Carry Handle for transporting unit. P. Anti-Glare Stand: This device is used to change the viewing angle of the NIM screen, it is shown in the tilted up position. Important: Intraoperative use of the VGA Out, and RCA Phone Jack requires special considerations to remain compliant with IEC/EN60601-1. Contact Medtronic Xomed for recommendations if intraoperative use of the VGA Out, RCA Phone Jack.
Patient Interface
A. Stimulator pads (Simulated Events). B. Stimulator return (anode) plug. C. Electrode ground plug D. Simulated subdermal electrode plugs.
Stimulator Probes/Handles
The Stimulator Probes and Handles carry stimulus current from the console, via the Patient Interface, to the patient.
Monopolar Ball Tip Probe
A. Stimulus to Patient Contact Area B. Insulated Sleeve C. Probe Base Standard Prass Flush Tip Probe
A. Stimulus to Patient Contact Area B. Insulated Sleeve C. Probe Base Universal Monopolar Probe Handle
A. Probe Jack B. Handle C. Stimulus Plug
Bipolar Side-by-Side Stimulating Probe
A. Patient Interface to console connector Aa. Connector release. B. Stimulating Instrument Jack or Stimulator Probes (Monopolar or Bipolar). C. Stimulus Return D. Electrode ground: signal return for patient electrodes. E. Patient Interface Clips. F. Negative Electrode Jacks: Negative electrodes have black wires and color-coded plugs. 8
A. Stimulus to Patient Contact Area. B. Insulating Sleeve. C. Stainless Steel Tubing. D. Cable Connection.
Nerve Integrity Monitor
Set‑Up Mode Prass Flush Tip Stimulating Probe EMG Endotracheal Tube: Contact electrodes designed to monitor both vocal cords.
Power Cords
A. Stimulus to Patient Contact Area. B. Insulating Sleeve. C. Stainless Steel Tubing. D. Cable Connection.
Muting Detector
See Precaution P8. The Muting Detector Probe is designed to detect the presence of electronic noise from external devices (such as electrocautery/ electrosurgical unit) that may cause interference on the EMG monitor. A
D
1897821 1895820 1895822 1895823
Power Cord, 6 Meter, 115 V Power Cord Standard Power Cord, Europe Power Cord, Japan, 100 V
The Splash Screen
E
B C
A. Anti-slide Ring. B. Insulating Sleeve. C. Ferrite D. Cable Connector. E. Electronic Noise Detection Area.
Electrodes
Electrode types recommended for use with the NIM 3.0 System Hookwire Electrode: Two small wires attached to the end of a hypodermic needle. Injected intramuscularly (then the hypodermic needle is removed) The wires are insulated to within 3 mm of the end and are designed to obtain a more specific response. Paired Subdermal Electrodes: Non-insulated high performance electrodes with 2.5 mm spacing.
Self Test
An internal integrity check is automatically performed each time the system is turned ON. (See Warning W8) On Power-up a series of messages are briefly displayed. Then the console does a series of self-tests on the hardware.
Set‑Up Mode Select Procedure Step 1 of 2 A
Prass Paired Electrodes: The electrodes are insulated to within 5 mm of the end with 5 mm spacing. Muscle-specific single use.
B
I
1. Select Procedure
H
Prass Paired Electrodes Small Hub: The electrodes are insulated to within 5 mm of the end with 2.5 mm spacing. Muscle-specific single use.
Setup
C J
Step 1 of 2
Monitoring
Information
Neuro/Otology Head/Neck
Custom Procedures
NIM-Pulse® 3.0
Subdermal Needle Electrodes: Non-insulated high performance electrodes 12 mm long with a 0.4 mm diameter. Electrode Ground (Green with Green Wire) and Electrode Stimulus Return (Red with White Wire): Always locate these electrodes in a non-innervated, electrically neutral area (electrically neutral areas are where the bone is close to the skin and the electrode will not contact muscle tissue). Ground should also be located between the stimulator and monitoring electrodes.
Nerve Integrity Monitor
G
D
Global Settings
Help
?
* indicates default settings have been changed 5/1/2009 9:00 AM
E
F
GUI vxxxx.x.xxxxx DSP vxxx.x.xx.xxxx
This is the default screen, requiring the operator to select an existing procedure or begin a new (custom) procedure. Optional: Operator may enter/change Date, Time, Language, or Data Fields via Global Setting button. A. Tool Bar: Used to navigate between Set‑Up and Monitoring environments. Note that Monitoring cannot be selected until a procedure has been selected. B. Set‑Up Button: Selected by default. C. Monitoring - not selectable at this operation. D. Help Button: See Help screen. E. Global Settings Button: See Global Settings screen. 9
Set‑Up Mode F. Time and Date Bar: This bar shows the time and date as set in the Global Settings panel. In addition it displays the GUI and DSP version. G. Print and Save Icon: These icons are displayed automatically (in all screens with a time and date bars) only if a USB drive and/or a Printer are connected. H. Select Procedure: Drop down menus. I. Set‑Up Wizard Navigation Bar. J. Information Button: See Case Information.
Global Settings
This panel is accessed by pressing the Global Settings button in any of the Set‑Up Mode Screens. Setup
A1. Select Procedure B
Language
Step 1 of 2
I
Global Settings
English
1/27/2007 11:34:06 PM
Neuro/Otology 1/27/2007 23:34:06
Italian
Head/Neck 2 7 / 1 / 2 0 0 7 2 3 : 3 4 : 0 6
Spanish
Opens automatically after selecting a factory installed procedure.
H
Set Date and Time
G
Diagnostic Mode
F
Restore Defaults
A B 2. Place Electrodes
Surgeon Patient Name
E
Notes
* indicates default settings have been changed
OK OK
?
Global Global Settings Settings
Help
Warning: EMG Monitoring Is Disabled
X
Date Format: 03/18/2008 Time Format: 09:24:34 AM Set Date/Time 03/18/2008
Date:
Time: 09:24:34 AM AM
PM
1
2
3
4
5
6
7
8
9
Clear
0
Ok
• Date entry fields for date and time. • AM and PM radio buttons. I. Date/Time Format: Used to select how date / time is displayed. The default format is sensitive to language.
Help Setup
1. Select Procedure Electrode Placement
E
1 - Orbicularis Oculi
?
2 - Orbicularis Oris
?
Stim 1 Return
?
Ground
?
GUI vxxxx.x.xxxxx DSP vxxx.x.xx.xxxx
A. Global Settings Panel. B. Language panel: Select language. C. Data Fields: Select the fields to be populated in the Case Information Screen. D. Blank data fields: Allows the operator to name two fields that will appear in the Case Information Screen. E. OK button will close Global Settings. F. See Buttons and Indicators. G. Diagnostics Mode: Not for end users. Should be used only under the direct supervision of Medtronic Xomed personnel. H. Opens a data entry key pad for setting the date and time.
D
Information
C
Step 1 of 2
Audio Samples
Show Details
11/4/2008 10:00AM
GUI vxxxx.x.xxxxx DSP vxxx.x.xx.xxxx
This screen will assist the operator with electrode location. It also runs an electrode check, this screen shows that electrodes are being tested. Note: If changes to the procedure are to be made and saved, then those changes must be made before selecting the Monitor or Monitoring button. Note: This screen can be bypassed by selecting the Monitor or Monitoring button. A. Tool Bar: Used to select any of the major (2) functional Modes. B. Set‑Up Wizard Navigation Bar. a. Previous: Returns to the Select Procedure screen. b. Monitor: Opens the Monitoring screen. See Monitoring Mode for details. Note: If changes were made in the Electrode Check panel, the Procedure Settings panel, or the Procedure Settings/Advanced Settings Tabs a dialog box will open asking if the operator wishes to save said changes. c. Information: Opens the Case Information screen. See Case Information for details. C. Electrode Check Tab: Closes/Opens Electrode Check panel. See Electrode Check Panels for details. D. Procedure Settings Tab: Opens/Closes Procedure Settings Panel. See Procedure Settings Panel for details. E. Electrode Placement Graphic: Shows electrode placement for both the patient and patient interface.
Electrode Check
Monitoring
B
Ω
D Procedure Settings
5/1/2009 9:00 AM
Nerve
Monitor
D
Patient DOB
C
Electrode Check
Step 2 of 2 Previous
Please Wait
NIM-Pulse® 3.0
M o n i t o r i ns tg P r o f e s s i o n a l
Patient ID
Diagram
Monitoring
2007/1/27 23:34:06
Custom Procedures
Data Fields for Case Notes
A
Setup
Mastoid
Electrode Check
French
Place Electrodes Step 2 of 2
Information
Date/Time Format
German
C
Monitoring
A. Electrode Placement: This tab selects help graphics for locating electrodes. B. Audio Sample: This tab enables sample three sound buttons: • Pulse • Train • Burst C. Diagram/Nerve: Radio button selects help graphics by nerve number/name. D. Diagram/Procedure: Radio button selects help graphics by Procedure. See Place Electrodes Step 2 for example. E. Previous Next Buttons: For changing graphics. F. Graphics display area. G. OK button will close Help screen.
Electrode Check, checks the integrity of the patient to Patient Interface connections. It is also the only location where electrode type in use can be changed.
Information
Neuro/Otology Head/Neck
Procedure
VII (2ch)
F
Custom Procedures
NIM-Pulse® 3.0
E
G * indicates default settings have been changed 5/1/2009 9:00 AM
OK OK ? ?
Global Settings
Help
Help Help
GUI vxxxx.x.xxxxx DSP vxxx.x.xx.xxxx
This screen will display help graphics for locating electrodes or sample audio sounds. 10
Nerve Integrity Monitor
Set‑Up Mode Electrode Check Panel
Electrode Check Show Details Panel
This panel can be accessed from two (2) locations: Setup
Mastoid 2. Place Electrodes
A
Step 2 of 2 Previous
Monitor
1 - Orbicularis Oculi
? ?
Stim 1 Return
?
Ground
?
E
Ω G
Electrode Check Electrode Check
8.1kΩ
Ground
6.6kΩ
Hide Details
Electrode Type Panel Electrode Check
Warning: EMG Monitoring is Disabled 1 - Orbicularis Oculi Subdermal
(+) 5.9kΩ (-) 5.9kΩ
∆ 0.0kΩ
(+) 6.0kΩType 22 -- Orbicularis OrbicularisOris Oris Electrode ∆ 48.2kΩ Subdermal
(-) 55.8kΩ
Subdermal Endotracheal Tube Hookwire Prass Paired Surface
Stim 1 Return
8.1kΩ
Ground
6.6kΩ 1.5kΩ 0.7kΩ
Hide Details
At this screen Radio Buttons are used to select the type of electrode being used (Subdermal by default).
Electrode Troubleshooting Guide Symptom Electrode impedance is too high. > 10 KΩ for subdermal electrodes > 10 KΩ for EMG tube Electrode impedance equals <0.1 KΩ
• • •
• •
•
Ground
Ω
Stim 1 Return
A. Electrode Type Button.
Electrode reading is • (+ or -) Off or • Δ==== • •
Stim 1 Return
Show Details
∆ 0.0kΩ
Electrode Check
The impedance values of the electrodes to the patient are measured to confirm the integrity of the connection.
1 - Orbicularis Oculi
(+) 6.0kΩ ∆ 48.2kΩ (-) 55.8kΩ
Subdermal
GUI vxxxx.x.xxxxx DSP vxxx.x.xx.xxxx
Electrode Check Panel Pass/Fail
2 - Orbicularis Oris
(+) 5.9kΩ (-) 5.9kΩ
2 - Orbicularis Oris
Show Details
A. Electrode Check Panel is typically displayed in the Set‑Up Mode with Place Electrodes Screen. It may be opened or closed at this screen using the Electrode Check Tab. Note: Changes made in the Set‑Up Mode can be saved. The only saveable change (saveable to a new or existing procedure) that can be made at this panel is the electrode type being used. This screen shows that a two (2) channel surgery (Mastoid) was selected. Channel 1 will be used to monitor Orbicularis Oculi and channel 2 Orbicularis Oris. B. Closes Electrode Check panel. C. Monitoring is disabled when the Electrode Check panel is open D. Electrode status field: • Progress bar: Displayed while electrodes are being tested. • Question Marks: Question marks are visible while the electrode test is running and will be replaced with pass (green check mark) or fail (red x) mark. E. STIM 1, STIM 2, and Ground status fields. Note: • There is no STIM status (blank) if Bipolar is selected in the Type Panel (located in the Advanced Settings/Stimulation Panel). • There is no STIM 2 status (blank) if a single stimulator is connected. • STIM 2 will be displayed after it is turned on using the Activate button located on the main screen or selecting the STIM 2 check box on the Procedure Settings/Stimulation Panel. F. Show Details button: See Electrode Check/Show Details. G. Print Button: Sends the electrode, ground, and STIM 1 & 2 impedance values to the Printer. Note: Print button is displayed only if a printer is connected.
Warning: EMG Monitoring is Disabled
1 - Orbicularis Oculi
Subdermal
Procedure Settings
2 - Orbicularis Oris
A
Electrode Check
Please Wait
D
Warning: EMG Monitoring is Disabled
Electrode Check
C
F
Electrode Check
Electrode Check Warning: EMG Monitoring Is Disabled
Information
11/4/2008 10:00AM
Press the Show Details Button to see the actual impedance values. See the Electrode Troubleshooting Guide in this section.
B
Monitoring
Cause Electrode dislodged • from patient, but not completely out. High resistance in • electrode. Electrode pin not firmly inserted into patient • interface. Positive and negative • electrodes touching below surface of skin. Extremely low impedance, particularly in EMG tubes. Electrode laying on skin • surface. Electrode placement insecure. • Dirty electrode tip. Electrode cable is broken. • Electrode pin disconnected from patient interface.
Solution Insert dislodged electrode; tape down in place. Remove and replace with new electrode. Check connection at Patient Interface box. Remove and relocate electrodes.
Re-insert electrode in question. Remove and replace electrode in question. Check connection to Patient Interface box.
This screen shows that channel 1, stimulus return, and ground electrodes have passed where channel 2 has failed.
Nerve Integrity Monitor
11
Set‑Up Mode
Case Information
Opens after pressing Information Button. A
Setup Mastoid Mastoid 1. Select Procedure
B Enter Case Information
Surgeon Patient ID
Monitoring
Step 1 of 2 Monitor
Previous
Information
Neuro/Otology Head/Neck
Patient Name
C
Custom Procedures
NIM-Pulse® 3.0
Notes
D Procedure Settings
E ?
* indicates default settings have been changed
Global Settings
5/1/2009 9:00 AM
Help
GUI vxxxx.x.xxxxx DSP vxxx.x.xx.xxxx
This screen is for data entry into preselected data fields. See Global Settings for data field selection. A. Tool Bar: Used to select any of the major (2) functional Modes. B. Set‑Up Wizard Navigation Bar: Previous, Monitor see Buttons and Indicators. C. Case Information: By pressing any of the information fields a keyboard for data entry will open. D. Procedure Settings Tab: Opens Procedure Settings panel. E. Global Settings, and Help Buttons: See Global Settings.
Procedure Settings Panel
3. Muscle Name: (1) Use the Scroll buttons to locate an existing muscle group. (2) Highlight (touch the screen) the muscle name. (3) Press OK to enter name and exit. Or (1) Key in (type) new muscle name. (2) Press OK to enter name and exit. B. Simulation Panel: Used to adjust STIM 1 stimulus and STIM 2.
Example shows panel with STIM 2 selected. C. Event Threshold Panel: Adjust the event threshold, the range is from 20 μV to 2500 μV in increments of 5 μV. D. Save Button:
This panel can only be accessed in the Set‑Up Mode Place Electrodes screen. A
Channels
a. Opens save option panel. b. Save option panel gives the operator the option to Overwrite the existing procedure, create a New procedure or Cancel. E. Advanced Settings Button: See Advanced Settings for details. F. Closes the panel.
1: Orbicularis Oculi 2: Orbicularis Oris 3: Inactive 4: Inactive 5: Inactive 6: Inactive 7: Inactive 8: Inactive
Advanced Settings
B Stimulation Stim1
30.0 2 mA
+
Stim2
Event Thershold 100 2 µV
D Save
E
+ Advanced Settings
Procedure Settings
C
F
Note: Changes made in this panel that can be saved (saveable to a new or existing procedure). If printing a snapshot it will include changes to: • Channels • STIM 1/2 • Event Threshold A. Channels: used to add, remove, or change the names of the channels 1. Press the Channel button, drop down list of channels will open.
Advanced Settings function: • Advanced Settings adjustments made in the Set‑Up Mode (Procedure Settings Panel), adjustments can be used for the current session OR saved. • Advanced Settings adjustments made in the Monitoring Mode (Control Panel) are effective only for the current session and CANNOT be saved. When the Advanced Settings button is pressed, a screen is opened with tabs that allow access to the functions described.
Audio Tab
Please review “Audio – Understanding What You Hear” with this section. Monitoring
Setup
147 µV
1 Vocalis Left
Thyroid
Ω
Stim 1
1.0 0.11 mA
Audio
Monitoring
+ Stim 2
B
Stimulus Delivery Audio
A Audio Settings C
Off
Monitoring Audio
Event Capture
EMG Audio
100
µV
Snapshot Action
Show Channel Mute Buttons
Voice - Settings
Comment
Threshold
+ Volume Balance
Snapshot
Volume 50
+
EMG Audio
100 µV
2
+
Event Tones
5/1/2009 9:00AM
3
+
Save
Control Panel
2. Press an active channel to inactivate that channel. Press an inactive channel to open the Muscle Name keyboard.
32
+
Event Threshold
55 µV
Voice - Stimulus
D
Measure
Event Tones
2 Vocalis Right
100 µV
Display
Events
Continuous Tone
Events
Freeze
Stimulation
Brief Tone Activate
Electrode Check
Voices
E OK OK OK
?
Advanced Advanced Advanced Settings Settings Settings
Help
50 mS GUI vxxxx.x.xxxxx DSP vxxx.x.xx.xxxx
A. Audio Settings: configures system to determine what sounds will be heard during monitoring and balance between various sounds. B. Stimulus Delivery Audio: The default setting is Brief Tone. Additional options: 12
Nerve Integrity Monitor
Set‑Up Mode •
Brief Tone (default): Delivery of stimulus current is accompanied by a brief warbled tone. • Continuous Tone: Delivery of stimulus current is accompanied by a continuous, warbled, high-low tone (referred to as “Stimulus Warble Tone”). • Voice - Stimulus: Delivery of current to the surgical field is announced by the word, “STIMULUS”. • Voice - Setting: Delivery of current to the surgical field is announced by the value of the stimulus setting. Note: Stimulus Delivery Audio is not heard when an event has occurred. C. Monitoring Audio: There are two options, EMG Audio and Event Tones. At least one selection must always be active however, both options may be selected. • EMG audio: is the amplified sound of muscle activity that is heard instantaneously as the nerve is stimulated. All EMG activity, regardless of amplitude, is audible when the EMG audio is ON. The EMG activity may sound like a low-pitched “drumbeat”, a high-pitched “crackle,” or a “growl”. When multiple channels are monitored, it is unlikely that you will be able to differentiate the EMG signals as to their channel of origin strictly by the sounds they produce. • Event Tones: are heard when the EMG amplitude is larger than the Event Threshold setting. The Event Tones are easily heard over O. R. noise and are heard at the same time with the EMG audio, previously mentioned. User can differentiate channels by tones pitch. The tone for channel 1 activity is lower in pitch than the channel 2 and so on for channels 3 through 8. When EMG activity exceeding the event threshold occurs at the same time on multiple channels, only two of the tones sound at once. User may allow individual EMG channels to be muted by selecting Show Channel Mute Buttons. D. Volume Balance: Sound levels can be adjusted using the + and – buttons for EMG Audio, Event Tones and Voices. A numeric value and white bar graph will indicate the setting relative to full scale (scale is 1 to 5). E. OK button will close Advance Settings panel.
Monitoring Tab Monitoring
Setup
147 µV
1 Vocalis Left
Thyroid
Ω
Stim 1
0.11 mA
Audio
Monitoring
+ Stim 2
View Scale
100000 µV
Monitoring Settings
A 0µV
1 Orbicularis Oculi
50000 µV
B
Events
2000 µV
C
2 Vocalis Right 0µV
2 Orbicularis Oris
D
Threshold
E
Volume 50
20 µV
25 mS
100 µV
50 mS
Measure
Event Capture
Sequence Display
100
Last
µV
55 µV
Snapshot Action
Stimulus 2.1 2 mS
100 mS
20 S
5/1/2009 9:00AM
+
Event Threshold
Comment
+
Rejection Period 50 mS
500µV 100µV
100 µV Snapshot
Display
Events
Auto Threshold
Largest Overall
100 µV1000 µV
+500 µV
Freeze
Event Capture
Activate
10000 µV
Electrode Check
Stimulation
Save
Waveform Filters Artifact Filter Low Frequency Filter
Control Panel
1.0
F OK OK OK Advanced Advanced Advanced Settings Settings Settings
? Help
50 mS GUI vxxxx.x.xxxxx DSP vxxx.x.xx.xxxx
A. View Scale, see Control Panel Display Button. B. Event Capture/Auto Threshold Check Box: If Event Tones are continuous for 10 seconds: • Auto Threshold will automatically calculate a new Event Threshold (to a maximum of 400 μV). • All activity less than the new Event Threshold will be heard as raw EMG. • EMG activity greater than the new Event Threshold or greater than 400 μV will generate Event Tones. • Additionally, if the Voice setting is selected, it will announce the amount of threshold increase. • The Event Threshold will return to the original value after 10 – 20 seconds of no, or decreased, event activity. C. Event Capture/Sequence Display/Last (default setting) • If Last and Event Capture (see Control Panel) are selected, the most recent event will be displayed until replaced with a new or more recent event.
Nerve Integrity Monitor
•
If Largest Overall and Event Capture (see Control Panel) are selected, and multiple events occur over a period of 4 seconds then the largest event from the series of events will be displayed as Largest x of x (example 3 of 5). • This will remain displayed until replaced with the next event or next largest event from a 4 second series of events. Note: that neither Event Capture nor Largest are available when the x-axis time scale is set to 10 seconds (it is only available in the 50ms time scale). D. Stimulus/Rejection Period, is an adjustable delay allowing the small amount of electronic noise caused by stimulation (Stimulus Artifact) to stabilize before reading EMG data.
Stimulus Artifact Example A. Original Rejection Period setting. B. Stimulus Artifact. C. Move Rejection Period line to here to avoid artifact. D. Trace as seen on Main Display. Note: In previous versions of the NIM, Stimulus Rejection Period was referred to as Stimulus Artifact or Artifact Delay. Special Note on Recognizing Artifact The NIM 3.0 System features sophisticated artifact rejection technology designed to provide highly sensitive and accurate monitoring. However, there may be electrically generated signals in the range of true response that the NIM 3.0 System cannot differentiate. Examples: • A transcutaneous stimulator used by the anesthesiologist might generate an audible signal. • Any external nerve locator/stimulator not synchronized (Muting Detector) with the NIM 3.0 System. • Electrical leakage from faulty thermal cautery units. You can identify the spurious signals by their lack of surgical context (there was nothing the surgeon was doing at that moment that could have caused a true EMG response). • If the recording electrodes and the stimulator (+) or (-) cables become tangled the resulting stimulus artifact might be spuriously detected as an EMG event. Be careful to route the recording electrodes away from stimulator cables. • The pace pulse generated by pacemakers may be detected and displayed by the NIM 3.0 System as a rhythmic artifact signal. This is caused by the electrode ground or stimulus return electrodes being in close proximity to the pacemaker or its lead wire(s). The artifact caused by the pacemaker may be reduced by repositioning the Electrode Ground and Stimulus Return electrodes to the top of the patient’s shoulder (the Acromion) (use shoulder opposite operated side). The Electrode Ground (green plug green wire) and Stimulus Return (red plug white wire) electrodes should be positioned about 5 cm apart, green Proximal, red Distal. Once the electrodes are repositioned, verify that the Stimulus Return and Impedances Ground are within tolerance (review Panels / Electrode Check ). • There may also be interference-generated signals in the range of true response that the NIM 3.0 System cannot differentiate. An example of this type of artifact signal could occur when the surgeon strikes two metal instruments together within the surgical field, such as a metal suction tube with a dissecting tool. Such signals are typically monophasic with fast onset and offset. That is, the signals appear on the screen as sharply peaked responses in one direction only. • While these artifacts are significantly different in waveform appearance from true EMG events (which have a biphasic waveform), the magnitudes of these signals can reach several hundred microvolts causing the event tone to sound. However, the surgeon is usually aware when two instruments have been struck together and can, therefore, relate such “false positive” responses to the surgical context.
13
Monitoring Mode E. Waveform Filters • Artifact Filter: Selecting this Check Box enables the detection of artifact as “Spiked Waveforms” (see Mechanical Stimulation in Appendix C) • Low Frequency Filter: Low Frequency Response is generally caused by the movement of the electrodes, electrode wires, tissue etc. This can result in a response that is not a true EMG response. Selecting this Check Box enables a filter that reduces Peak to Peak amplitude (about 20 %) in a frequency range below 70 Hz reducing unwanted response. The filter is on by default for all procedures. F. OK button will close Advance Settings panel.
Stimulation Tab Important Note on Stimulator Adjustments The absolute stimulus intensity required to adequately stimulate any motor nerve is determined by a complex combination of several factors including (but not limited to) the following: • The functional health of the nerve itself. • The type of stimulation probe used (monopolar or bipolar). • Proximity to the nerve. • The pulse width of the stimulus. You should use the smallest amount of stimulus necessary to elicit a detectable EMG event. Stimulus current levels of 0.3 mA may be high enough for adequate direct monopolar stimulation of the facial nerves, but may elicit little or no response when monitoring motor evoked potentials. The best guideline for setting the stimulus intensity level is to use the lowest amount that produces an EMG event. The surgeon should be aware: • That STIMULUS is continuously being applied to the STIMULATOR probe. • STIMULATING the patient is the result of physical contact between the patient and the STIMULATOR probe. • The STIMULATOR probe should be kept ISOLATED when NOT STIMULATING the patient. Note: Stimulator adjustment involves the adjustment of the Current, Rate, Pulse Width, and the Stimulus/Rejection Period. Note: To prevent inadvertent high stimulus levels, the first time the stimulus level exceeds 3 milliamperes, a dialog box will open: STIMULUS LEVEL WARNING Stimulus in excess of 3 milliamperes Press OK to allow stimulus If using the procedures Lower Extremity, Knee, or Ankle the stimulus level warning is set at 12.0 milliamperes. Monitoring
147 µV
1 Vocalis Left
Thyroid
1.0 Audio
Monitoring
0.11 mA
+
Stimulator 2
Stimulator 1 Name
Name
Stim 1
Stim 2Type
B C
Bipolar
Rate Activate 1/S
Events
100 µV Threshold
D Pulse Width
50 µS
2 Vocalis Right
E
Volume 50
µV
4/S
100 µS
7/S
150 µS
10/S
100 µV
3.0 2 mA
Current Warning Level
5/1/2009 9:00AM
Comment
0.11 mA
E
147 µV
M O 2 Vocalis Right
Events
100 µV
P
L
55 µV
Threshold
+
F
Advanced Advanced Advanced Settings Settings Settings
P
Stim 2
Save
OK OK
C
Activate
F
? Help
50 mS
K
M
G Snapshot
N
Volume
GUI vxxxx.x.xxxxx DSP vxxx.x.xx.xxxx
A. Name/text field, pressing the text field will open the Stimulator 1 or 2 Name keyboard.
14
1.0
Snapshot Action
200 µS 250 µS
+
Monitoring
Setup
L
Event Threshold
55 µV
150 µS
Current Warning Level
D Stim 1
+
Event Capture
100
100 µS
3.0 2 mA
Measure
50 µS
1/S
250 µS Snapshot
Display
Pulse Width
Rate
7/S
200 µS
Freeze
Bipolar
Monopolar
4/S
+ 10/S
Electrode Check
B 1 Vocalis Left
Thyroid
Events
Type Monopolar
This screen is displayed by selecting the Monitor or Monitoring button. It is the screen displayed during surgery and shows all EMG activity.
Stim 2
Control Panel
A
Stimulation Stimulation Stimulation Settings Settings
Monitoring Mode A
Ω
Stim 1
A Pulse Width (duration time of each pulse) B Rate (number of pulses per second) E. Maximum Current Setting used to adjust stimulator current if being accessed from Set‑Up/Procedure Settings/Advanced Settings. No adjustment is available if accessed from Monitoring/Control Panel/ Advanced Settings. F. OK button will close advance settings panel. Repeat for Stimulator 2 panel.
H 50
I
100 µV
N 5/1/2009 9:00AM
J
Control Panel
Setup
a. Use the Scroll buttons to locate an existing probe. b. Highlight (touch the screen) the probe name. c. Press OK to enter name and exit. Or a. Touch screen at Stimulator (1 or 2) Name. b. Key in new name. c. Press OK to enter name and exit. B. Type Panel is used in the selection of monopolar or bipolar probes. By default monopolar is selected. If bipolar probes are being used the operator must change probe type at this location. C. This panel is used for making adjustments to the stimulus Rate . D. This panel is used for making adjustments to the stimulus Pulse Width.
50 mS GUI vxxxx.x.xxxxx DSP vxxx.x.xx.xxxx
A. Tool Bar: Used to select any of the major functional Modes. B. Set‑Up Button: opens the Set‑Up Screen. C. Monitoring Button: Opens the Monitoring Screen. D. STIM 1 Panel: Displays the stimulation setting (large numbers), the measured value (in the small window), and adjustment buttons for the STIM 1 current settings. E. STIM 2 Panel: If inactive it will display the “Activate” button. If active as a second stimulator it’s display is the same as STIM 1. F. Events Panel: This panel displays the Event Threshold settings and allows the operator to adjust the setting level (in 5 μV increments). • EVENT THRESHOLD is a highly sensitive filter used to define where EMG activity becomes significant. • EMG activity exceeding this “THRESHOLD” is defined as an “EVENT” and results in Event Tones (alarms) sounding. Nerve Integrity Monitor
Monitoring Mode •
Control Panel
The Control Panel, is used for additional settings and monitoring features. Monitoring
Setup
147 µV
1 Vocalis Left
Stim 1
1.0
B A
0.11 mA
+
Setup
1.0
D
Display
Measure
100000 µV
+ Stim 2
a Activate
2 Vocalis Right
Threshold
Activate
Events
Snapshot Action
100 µV
Comment
Threshold
+ Control Panel
5/1/2009 9:00AM
A. Display Button, opens the Scale Panel.
50 mS
25 mS
50 mS
100 mS
20 S
Print Save
?
F
GUI vxxxx.x.xxxxx DSP vxxx.x.xx.xxxx
a. The vertical portion of the EMG display represents Amplitude and the scale is adjustable (500 μV by default). The vertical screen is divided into equal sections per channel with 1/2 of each channel positive and 1/2 negative. Each channel is separated by a solid blue line. b. Time scale is represented by the horizontal portion of the EMG display and is adjustable (25mS by default) (see Advanced Settings / Monitoring Tab). The screen is divided into equal sections. This differs from the “Amplitude scale” in that the “Time scale” selection is the entire horizontal portion of the screen. c. OK Button, closes Scales panel. Note: Changing either scale only affects how the data appears on the screen. It does not modify the sensitivity of the unit. B. Electrode Check: See Electrode Check Panel. C. Freeze: Pressing the “Freeze” button causes the screen contents to be displayed, unchanged, until the next press of the Freeze button “unfreezes” the display. This is useful to view an event over an extended period. Note: Any events that take place when Freeze is on are not displayed. D. Measure Button: for viewing details of an event waveform. Setup
Monitoring
347 µV
1 Vocalis Left
Stim 1
1.0
Ω
147µV, 5.25mS
b
Stim 2
c
5.25
d
Events Cursor
Event Capture
µV
+
Event Threshold
55 µV
Snapshot Action Comment
a
Threshold
27µV, 5.25mS
Save
Help
H
Measure Measure
100
?
G
OK OK
mS Display
Activate
100 µV
GUI vxxxx.x.xxxxx DSP vxxx.x.xx.xxxx
Freeze
Electrode Check
a
+
Advanced Settings
Help
Advanced Settings
50 mS
5/1/2009 9:00AM
2 Vocalis Right
Save
100 µV
Snapshot Action Comment
Events
Snapshot
50 mS
500µV
Event Threshold
Event Threshold
55 µV
2 Vocalis Right
µV
55 µV 50 mS
+
+ E
+
100
b
100 µV
Event Capture
0µV
2 Orbicularis Oris
1000 µV
Snapshot
Volume 50
Measure
c
20 µV
+
Event Capture
µV
Scales Scales Display
Events
2000 µV
Freeze
OK OK
10000 µV
100 µV
100 µV
Events 100
0µV
1 Orbicularis Oculi
50000 µV
500 µV
Events
0.11 mA
Stim 2
Volume 50
Electrode Check
0.11 mA
Thyroid
Freeze
Ω
Stim 1
C
Ω Electrode Check
147 µV
1 Vocalis Left
Thyroid
Control Panel
Thyroid
Monitoring
Control Panel
Set “Event Threshold” by pressing plus/minus buttons to increase or decrease the value as desired. Setting is displayed in micro‑Volts. a. If the Event Capture is turned on (default) the Snapshot Button will be visible. See Appendix D for information on the Snapshot Button. G. Snapshot Button: Sends current screen to selected peripheral device: • Comments Icon Snapshot Option - See Control Panel. • Print and Save Icon Snapshot Option These icons are displayed automatically if a USB drive and/or a Printer are connected - See Control Panel. Note: Snapshots (print or save) show event information only and will not include the impedance values of the electrode, ground, or STIM 1/2. H. Volume Panel: Displays adjustable volume setting. Sound levels can be adjusted using the volume knob (see Console Front, Item C). A numeric value and white bar graph will indicate the setting relative to full scale. The default setting is 50. I. Print and Save Icon: These icons are displayed automatically (in all screens with a time and date bars) only if a USB drive and/or a Printer are connected. J. Date, Time, GUI, and DSP version. K. Control Panel Tab: Opens Control Panel. L. Channel Label: Displays the channel number and nerve being monitored. M. Trace: Displays stimulus nerve activity/inactivity. N. Scale: Displays screen scale settings. O. Artifact Delay: Shows where artifact ends and EMG begins. P. Amplitude: Displays in microvolts activity level on each channel: • A box will enclose the activity level of any channel showing an event. • If there are multiple channel events, the box will enclose the largest. • If the system detects a signal (response) that is outside the range of the systems ability to measure (80 - 100,000 μV or higher) “Out of Range” will be displayed.
Snapshot
Volume 50
100 µV
5/1/2009 9:00AM
? Advanced Settings
Help
50 mS GUI vxxxx.x.xxxxx DSP vxxx.x.xx.xxxx
a. The on screen display (green) will show the amplitude (μV) and time (mS) at the cursor location. • Time is measured from zero (0) not from Stimulus/Rejection Period. • Amplitude is measured from the channels zero (0) with a minus (-) sign for negative numbers and no minus sign for positive numbers. b. The time will also be shown in the display. c. Cursor Position buttons will move the vertical (green line) cursor left or right in increments of 0.25 mS. d. The cursors and displayed will turn off when the measure OK button is pressed. E. Events/Event Capture: Captures and holds EMG activity that exceeds the EVENT THRESHOLD. F. Events/Snapshot Action:
Nerve Integrity Monitor
15
Special Functions and Features •
Comments: Opens comments panel allowing comments to be added to the captured event when the Snapshot button is pressed. These comments will be displayed on the printed and/or saved event. • Print: Sends captured event to the printer with comments if selected when the Snapshot button is pressed. • Save: Sends captured event to the USB mass storage device with comments if selected when the Snapshot button is pressed. G. Advanced Settings button: Opens Advanced Settings. See Set‑up Mode/Advanced Settings for information. Adjustments made to the Advanced Settings in the Monitoring Mode (Control Panel) are effective only for the current session and CANNOT be saved. H. Help: Is available as abbreviated assistance on the Monitor Display.
Special Functions and Features Visual Alarms and Warnings
The operator may encounter the following warnings and should respond appropriately.
Audio – Understanding What You Hear
The NIM 3.0 System Nerve Monitor produces many different sounds throughout surgical procedure that require understanding by the person performing monitoring. In addition to EMG sounds, various beeps and voices provide useful information.
Alarms
The alarms draw attention to any condition, which prevents proper monitoring. None of the audible alarms should be ignored. One must assume that valid monitoring has stopped and determine immediately why the alarm sounded. There are three distinct alarms: • BEEP Alarm: The high-pitched, repetitive beep is used to register failure of internal microprocessor hardware. • If you hear it at any time other than at power up, stop using the NIM 3.0 System and contact Medtronic. See Customer Care section. • Bleedle Alarm: This is a three-note alarm (Blee-Dle Deet) that cannot be disabled. It repeats until the responsible condition is remedied. The alarm is heard once under the following conditions: • Check Electrode • Muting For more information on these two conditions please see Special Functions and Features / Muting conditions. • Voice Alarms: There are two voice alarms that are selectable and work in unison with the bleedle alarm. They can be turned on or off and have volume controls. These alarms are “check electrodes” and “muting,” and are described in System Set‑Up.
Voice Annunciation
There are several voice annunciation types, stimulation delivery confirmation, numeric stimulator current values, and activation of auto threshold. NIM® 3.0 System Voices Point Five Threshold increased Zero Six Check electrodes One Seven Electrodes Two Eight Stimulus Three Nine Four Threshold decreased Threshold / Voice These voices are used as part of the Auto Threshold feature when automatic adjustments are made. Please see the Auto Threshold section of the manual. The NIM® 3.0 System has two (2) Threshold voices: “Threshold increased” - “Threshold decreased”. Help / Voices “Check electrode” is announced when contact with an electrode is lost.
Stimulus Delivery Audio Tone The stimulus tone announces the delivery of current to the patient. • If Continuous Tone is used, then delivery of stimulus current is accompanied by a continuous, warbled, high-low tone (referred to as “Stimulus Warble Tone”). • If Brief Tone is used, then delivery of stimulus current is accompanied by a brief warbled tone. Note: Stimulus tones will not sound for stimulus current levels below 0.05 mA. Stimulus tones are not heard if an Event Tone has been generated. Voice The stimulus voice announces the delivery of current to the surgical field by voice. • Voice - Stimulus: With “Stimulus”, delivery of current to the surgical field is announced by the word, “STIMULUS”. It may be turned on or off and has volume control. • Voice - Setting The “Setting” voice announces the value of the STIMULUS setting. It also announces any new stimulus setting when adjustments are made. Example: 0.50 mA would be announced as “Point Five”. It may be turned on or off and has volume control. Note: Voice - Setting or Voice - Stimulus will not sound for stimulus current levels below 0.05mA. Stimulus voices are not heard if an Event Tone has been generated. 16
Nerve Integrity Monitor
Other EMG Responses (Samples are available in the Help Screen). • Burst - Brief tone: Caused by electrical stimulation, direct nerve contact, irrigation, or thermal changes. • Train Tones lasting seconds to minutes: Caused by nerve excited/ irritated, irrigation, drying, bumping, or anesthesia. • Pulse - Repeated tones: Caused by electrical stimulation, tumor mapping, verifying nerve integrity.
Muting
The Muting Detector is normally clamped around the active cable of an electrosurgical instrument that would otherwise be interfering with monitoring. Do not include the grounding pad cable in the jaws of the Muting Detector. It may also be connected to other equipment cables, such as electrocautery, ultrasonic debulking devices (e.g. CUSA), or other external devices, which generate interfering signals. Always attach the Muting Detector close to the output jacks of the electrosurgical or other unit rather than near the hand piece the surgeon uses. More than one output cable may be clamped within the Muting Detector jaws at one time. Monopolar Electrosurgical Instrument Clamping The power level for satisfactory muting performance of electrosurgical unit. Monopolar Electrosurgical Unit 5-100 W
System Set‑up
System Set‑up
Operating Room Set‑up
The NIM 3.0 System Nerve Monitor has a few characteristics that need to be taken into consideration when setting up the OR. Some of these characteristics include, but are not limited to, external devices, traffic patterns, sterile areas, color-coding, grounding, and muting detection. Set the NIM 3.0 System on a table or cart located about 3 meters from the surgical field but as far as possible from the electrosurgical unit. Also, consider traffic patterns and sterile areas. The surgeon may have further preferences as to location and visibility.
Typical Set-Up (shown with IPC™)
Muting Conditions Check Electrode: As soon as contact with an electrode is lost “Artifact Detected” is displayed on the zero (0) amplitude line in yellow There is a continuous noise due to ambient electronic noise being picked up by the disconnected electrode. After 3 seconds the noise stops and “Electrode Off ” is displayed on the zero (0) amplitude line in yellow with the background noise waveform The bleedle alarm will repeat every 20 to 30 Seconds until the condition is remedied. Muting from External Source: As soon as the Muting Detector senses current flow, the dialog box “ Monitoring/WARNING EMG MONITORING IS DISABLED/Muting from external source” and the system will be in mute. After 20 to 30 seconds, the bleedle alarm will sound followed by the “Muting” voice. The bleedle alarm will repeat every 20 to 30 Seconds until the condition is remedied.
STIM Bur Guard
A. Anesthesia Equipment B. IPC™ System or XPS® 3000 (REF 1897102BF only) if used C. Nursing Supplies / Surgical Instruments D. Scrub Nurse E. NIM® Monitor F. Microscope G. Surgeon H. Electro Surgical Unit I. Anesthesiologist
Anesthesia Requirements
All decisions regarding anesthesia are the responsibility of the attending licensed medical practitioner administering the anesthesia. Because all intraoperative monitoring discussed in this User’s Guide requires that EMG activity be recorded from a muscle or muscles, it is important that the muscle(s) not be paralyzed during the surgery or at least during those parts of the surgery when the nerve(s) being monitored is (are) deemed at risk by the surgeon. It is important that the surgeon discuss these issues pre operatively with the attending licensed medical practitioner administering the anesthesia. Note the following Contraindications for Use: The use of paralyzing anesthetic agents will significantly reduce, if not completely eliminate, EMG responses to direct or passive nerve stimulation. Whenever nerve paralysis is suspected, consult an anesthesiologist.
Muting Detector Set‑up The STIM Bur Guard provides stimulating current to standard Medtronic burs in both static and dynamic modes. The STIM Bur Guard locks onto any Visao® handpiece, while the cable connects to a port on the IPC™ System or XPS® 3000 (REF 1897102BF). A separate cable then connects to the NIM® Patient Interface Box. Wires within the STIM Bur Guard make contact with the uncoated bur and carry the stimulating current to the bur tip. Prior to using this device, the user must be familiar with the NIM® Systems User’s Guide, the Bur manual, the STIM Bur Guard User’s Guide, and the IPC™ System or XPS® 3000 (REF 1897102BF) User’s Guide. Compatible equipment: • XPS® Console (1897102BF) • IPC™ Console For more information related to the STIM Bur Guard system contact your local Medtronic representative
See Precaution P8. 1. Open the Muting Detector jaws and insert the cable from the electrosurgical instruments, ensuring that the wire is free to move and the jaws are completely closed. 2. Route the wire straight through the clamp.
Standard clamping Nerve Integrity Monitor
17
Patient Interface Set‑up Two electrodes per monitoring channel and one ground are required. The electrode pairs for each channel plug into color-coded input jacks on the patient interface. Route the white (+) stimulus return wire (monopolar) away from the channel electrode wires. If/when wires must cross; they should cross at right angles. Never run other operating room cords/cables parallel to any of the NIM 3.0 System wires. Verify the electrodes are properly inserted by turning on the NIM 3.0 System and looking at the Electrode screen. This may determine if electrodes need to be re-inserted for better electrical contact to help optimize monitoring. Lay the Patient Interface cable out of busy traffic patterns and secure it to the floor as needed. Monopolar and Bipolar (if needed) Clamping Note: Bipolar clamping is normally not needed but if power level is very low it may be necessary to loop the single conductor around and through the clamp see instruction 7 for clamping instructions. 3. Slide the cable so the probe is near the main unit of the electrosurgical instruments, not near the hand piece(s). 4. Slip the upper side of the anti-slide ring around the end of the Muting Detector to form a “U” around the cable. Pull it snugly against the probe’s lower jaws (see Steps 1 & 2).
Patient Interface and Single Stimulators
The NIM 3.0 System Stimulator output supports the use of both monopolar and bipolar stimulating probes with a range of 0-30 mA.
Note: The NIM-Pulse® 3.0 Patient Interface shown here is for reference only. The NIM-Pulse® 3.0 Patient Interface is used in the illustrations in this section. ALL connections are the same.
Monopolar Probe with Universal Handle F Step 1 Step 2 5. Do not clip the Muting Detector to the grounding pad cable. 6. The Muting Detector and Patient Interface cables should be secured to the floor with tape or other non-slip/trip device.
B C D A
E
G
7. For very low power levels it may be necessary to loop the single conductor around and through the clamp once.
Patient Interface Set‑up
Prior to surgery, the Patient Interface and Stimulator must be setup. This may be done prior to the NIM 3.0 System being turned on, or the NIM 3.0 System may be used to support the process of determining which nerves to monitor and positioning the electrodes appropriately (see Electrode placement in Special Functions and Features). Clip the Patient Interface near the surgical site within reach of the sterile electrode leads and sterile stimulator cable(s). Position it so that it is out of the way of the doctor and the scrub nurse. Placement of electrodes should be performed by or under the direction of a licensed medical practitioner who determines the particular nerves that are at-risk with each particular patient and procedure. The physician then places electrodes in the muscles innervated by the nerves at-risk. The electrode sites should be cleaned with alcohol to remove oils from the skin. The electrode needles are inserted into the patient, taped into position, and the wires routed away from where the surgeon will be working. Usually, recording electrodes are placed before the sterile field is draped and defined. Never let electrode leads contact one another.
18
A. Paired Electrodes. B. Negative Electrode Jacks (electrode plug matches color and wire is black). C. Positive Electrode Jacks (electrodes plug and wire are the same color). D. Electrode Ground. E. STIM 1 Stimulator Return. F. Stimulator Output, Monopolar Probe and Universal Handle (cathode). G. Operated Side. The patient interface supports one or two single use probes. The handle plug is color-coded black and plugs into the black (-) jack on the patient interface. The monopolar stimulating probe delivers current to the patient and acts as the cathode. The red electrode with white wire (+) is required for monopolar stimulation. It is referred to as the stimulus return or anode. Plug the red connector of the red electrode with white wire into the white jack with a red ring (+). Route the white (+) stimulus return wire away from the channel 1-2 electrode wires.
Nerve Integrity Monitor
Patient Interface Set‑up Bipolar Probe
Monopolar Probe and Stimulus Dissection Probe
H G
E F
H
B C
G
B D
C
A
D E
A
F
H
A. Paired Electrodes. B. Negative Electrode Jacks (electrode plug matches color and wire is black). C. Positive Electrode Jacks (electrodes plug and wire are the same color). D. Electrode Ground. E. STIM 1 Stimulator Return (anode). F. Stimulator Output (cathode). G. Single Use Bipolar Probe and Handle. Note: By default Monopolar (STIM 1 and STIM 2) is selected in the Type Panel (located in the Advanced Settings/Stimulation Panel). The operator should change this to Bipolar. H. Operated Side. The stimulator connection uses two different diameter pins to connect to the cable. The cable to patient interface uses color-coded connectors (Green wire with a red plug, connecting to the white jack (+) with a red ring, and black plug connecting to the black (-) jack).
Patient Interface and Stimulator Combinations
Note: • In previous versions of the NIM® STIM 1 and STIM 2 were wired in parallel (any signal applied to one was applied to both). The NIM® 3.0 STIM 1 and STIM 2 are wired independent of each other with each requiring an output (stimulator) and a return (red electrode with white wire). • By default STIM 1 is on and STIM 2 is off. STIM 2 must be turned on manually. See Monitoring Mode or Set‑Up Mode. • By default STIM 1 and 2 are Monopolar Probes and must be changed manually for Bipolar. See Advanced Settings.
I A. Paired Electrodes. B. Negative Electrode Jacks (electrode plug matches color and wire is black). C. Positive Electrode Jacks (electrodes plug and wire are the same color). D. Electrode Ground. E. STIM 2 Stimulator Return (anode) Probe. F. Stimulator Output, Monopolar Probe with Universal Handle (cathode). G. Stimulator Output, Stimulus Dissection Probe and Cable (cathode). H. STIM 1 Stimulator Return (anode). I. Operated Side.
Bipolar and Monopolar Probe I H G
E F
B C D A
I
J H A. Paired Electrodes. B. Negative Electrode Jacks (electrode plug matches color and wire is black). C. Positive Electrode Jacks (electrodes plug and wire are the same color). D. Electrode Ground. E. STIM 1 Stimulator Return (anode). F. Stimulator Output (cathode). G. Bipolar Probe and Handle. Note: By default Monopolar (STIM 1 and STIM 2) is selected in the Type Panel (located in the Advanced Settings/Stimulation Panel). The operator should change this to Bipolar. H. Operated Side. I. Stimulator Output, Monopolar Probe with Universal Handle (cathode). J. STIM 2 Stimulator Return (anode) Probe. Nerve Integrity Monitor
19
Monitor Set‑up
Monitor Set‑up
The NIM 3.0 System has a large number of user selectable characteristics used during a typical setup procedure. This section will address the three most common types of setup procedures: • “Basic” Set‑up. Basic information used by all programs i.e. Time and Date. • “Standard” Set‑up that uses a factory loaded program. • “Custom” Set‑up. This allows the surgeon to define the surgery being performed and the nerves to be monitored.
Basic Set‑up All Procedures
Read this section while viewing “Set‑up Mode”. 1. Connect unit to AC power. 2. Turn power switch to the On position. 3. After self test is complete press the “Global Settings” button. 4. Select Language (English is default). 5. Select “Time/Date Format”. 6. Press “Set Date and Time” button keypad will open. 7. Touch the text field for “Date:” and using keypad buttons enter date. 8. Touch the text field for “Time:” and using keypad buttons enter time. 9. If formatted for AM/PM then select AM or PM. 10. “Clear” button will clear selected text field, “Apply” button will enter new time and date, “X” button will exit keypad. 11. In the “Data Fields for Case Notes” there are six (6) selectable predefined text fields and two (2) user definable text fields. I. Select any combination of “Data Fields”. II. Pressing on the text field (<title>) will open a keyboard to enter a “Title” for the user definable text field. 12. Press “OK to close “Global Settings Panel”.
Standard Set‑up
Read this section while viewing “Set‑up Mode, and Patient Interface Set Up”. 1. “Select Procedure” screen. I. “Neuro/Otology, Head/Neck, and Peripheral” each open drop‑down menus of predefined procedures. II. Select by pressing the desired procedure. 2. At this point the “Place Electrode” screen will open. Note: The console checks for the presence of electrodes during the power up sequence. If electrodes were connected prior to powering up then skip this step. I. If patient interface is not connected then connect it now (see Console Rear). II. Connect electrodes according to graphic display (both patient interface and patient). III. Connect stimulation probe (see Patient Interface Set Up). 3. Review the “Check Electrodes” panel. All electrodes including STIM 1/2 and ground have green check marks. If not see “Electrode Troubleshooting Guide”. 4. To change electrode type: I. Press the “Show Details” button. Each channel will show a button with the muscle group being monitored and the electrode type. II. Press the channel button. A drop-down menu will open showing available electrodes. III. When you select the electrode the menu will close. 5. In the “Place Electrodes” tool bar press the “Next” button. 6. “Enter Case Information” screen will open. I. Press on the desired text field. II. A keyboard will open for entering text. III. When text has been entered press “OK”. IV. Continue in this fashion until all text fields are complete. 7. Press the “Monitor or Monitoring” button.
II. Connect electrodes according to the surgeons directions as the graphic display is blank/not helpful. III. Connect stimulation probe (see Patient Interface Set Up). 7. Review the “Check Electrodes” panel. I. To change electrode type: i. Press the “Show Details” button. Each channel will show a button with 1-Channel 1, 2-Channel 2 etc. and the electrode type as subdermal. ii. Press the numbered channel button. A drop-down menu will open showing available electrodes. When you select the electrode the menu will close. All electrodes including STIM 1/2 and ground have green check marks. If not see “Electrode Troubleshooting Guide”. 8. Press the “Check Electrode” tab. The electrode panel will close. 9. Press the “Procedure Settings” tab. The procedures setting panel will open. 10. To assign muscle group names to the channels: I. Press the “Channels” button. II. Channels can only be named when they are “Inactive” press any active channel once to inactivate it. III. Press the “Inactive” button. Keyboard will open. IV. Enter channel name. V. Press OK. VI. When all channels are complete press the “Channels” button to close drop-down menu. Note: At this point the operator may choose to save this procedure or see “Additional Settings”.
When the Case is Complete
Before closing the surgical site, the surgeon should evaluate the nerve’s functional integrity one more time. This can be done by stimulating the nerve both proximal and distal to the immediate dissection area as far as is accessible within the surgical field. Use the lowest level of stimulation that produces a response. Continue to monitor with the NIM-3.0 System throughout the procedure (until the incision is closed), since items such as wound dressings might exert stress or pressure on the nerve and affect its function.
When Monitoring is Complete
Turn OFF the power to the NIM-3.0 System when the entire procedure is completed, and have the licensed medical practitioner remove the electrodes from the patient. Contaminated single use electrodes and probes must be disposed of in an appropriate sharps biohazard container in accordance with hospital or other user facilities policy.
Power Disconnection
To disconnect equipment from the power, remove power cord from the supply receptacle. Note: All stored data (Snapshots) is lost when unit is powered off.
Custom Set‑up
Read this section while viewing “Set‑up Screens, Panels, and Patient Interface Set‑Up”. 1. “Select Procedure” screen press “Custom Procedure”. 2. Drop‑down menu will open, press “New”. 3. Keyboard will open, enter name of custom procedure. Press “OK”. 4. The new procedure will appear as a button in the “Custom Procedure” drop‑down menu. 5. Press the new procedure button. 6. At this point the “Place Electrode” screen will open. Note: The console checks for the presence of electrodes during the power up sequence. If electrodes were connected prior to powering up then skip this step. I. If patient interface is not connected then connect it now (see Console Rear”. 20
Nerve Integrity Monitor