ndd Medical Technologies
EasyOne Pro-LAB Operators Manual Rev V5.0
Operators Manual
171 Pages
Preview
Page 1
new diagnostic design
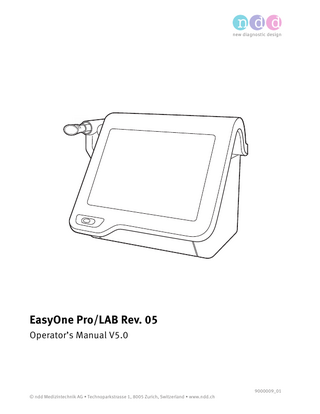
EasyOne Pro/LAB Rev. 05 Operator’s Manual V5.0
© ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
9000009_01
Preface
1
Preface
1.1
Revision history of the EasyOne Pro/LAB Operator’s Manual
Revision Date
Version
Description
22 February 2017
V5.0
Newly created edition for major new hardware revision Rev. 05 of EasyOne Pro/LAB
Revision history of the EasyOne Pro/LAB Operator’s Manual
1.2
Identification and revision of the EasyOne Pro Respiratory Analysis System This revision V5.0 of the EasyOne Pro/LAB Operator’s Manual applies to EasyOne Pro Respiratory Analysis Systems with a revision number greater than Rev. 05. If you are in doubt whether this revision of the Operator’s Manual applies to your particular EasyOne Pro/LAB, please contact the ndd Servicing Department. You can find the most recent revision of this Operator’s Manual on the ndd website. Contact information, 4 On www.ndd.ch For the US on www.nddmed.com
1.3
Version history of the EasyOne Pro/LAB firmware A comprehensive version history of EasyOne Pro/LAB is available on the ndd website. Simply search for “version history” on the ndd website. On www.ndd.ch For the US on www.nddmed.com
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
2/171
4
Preface
1.4
Intended use of the EasyOne Pro Respiratory Analysis System EasyOne Pro/LAB is designed for conducting lung function measurements in general or specialist practices or in hospitals. EasyOne Pro/LAB can also be used outside of the laboratory when performing lung function screenings or measurements in occupational medicine. EasyOne Pro/LAB is used to conduct lung function measurements on adults and children starting at age 4, except measurements of diffusing capacity of the lung based on CO (DLCO), which can be performed on adults and children starting at age 6.
1.5
Intended audience of this Operator’s Manual This Operator’s Manual is intended for general practitioners, specialists, and health care professionals. General practitioners, specialists, and health care professionals are expected to have working knowledge of medical procedures, practices, and terminology as required to conduct or interpret diagnostic spirometry tests.
1.6
Using this Operator’s Manual Read this Operator’s Manual prior to operating EasyOne Pro/LAB. Store the Operator’s Manual in a safe and easily accessible place.
1.7
Application Notes for further information You can find further information on specialized topics in Application Notes on the ndd website. http://AppNotes.ndd.ch For the US http://AppNotes.nddmed.com
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
3/171
4
Preface
1.8
Legal information Due to continuing product innovation, specifications in this manual are subject to change without notice. © ndd Medizintechnik AG, Zurich, Switzerland. All rights reserved. No part of this manual may be reproduced without written permission from ndd. ndd, ndd new diagnostic design and EasyOne are registered trademarks of ndd Medizintechnik AG. PCL® is a registered trademark of Hewlett-Packard Development Company, L.P. Wi-Fi® is a registered trademark of Wi-Fi Alliance. Microsoft and Windows are either registered trademarks or trademarks of Microsoft Corporation in the United States and/or other countries.
1.9
1.10
Contact information ndd Medizintechnik AG
ndd Medical Technologies
Technoparkstrasse 1
300 Brickstone Square, Suite 604
CH-8005 Zurich, Switzerland
Andover, MA 01810, USA
Tel: +41 44 445 2530
Tel: +1 978 470 0923
Fax: +41 44 445 2531
Fax: +1 978 470 0924
www.ndd.ch
www.nddmed.com
Product registration Registering EasyOne Pro/LAB facilitates the handling of warranty claims. To register EasyOne Pro/LAB, go to the ndd website. http://reg.ndd.ch For the US http://reg.nddmed.com
1.11
Disposal In the European Union, the product you have purchased should not be disposed of as unsorted municipal waste. Please make use of your local WEEE collection facilities to dispose of this product and otherwise observe all applicable requirements.
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
4/171
4
Table of contents 1 Preface
2 Revision history of the EasyOne Pro/LAB Operator’s Manual... 2 Identification and revision of the EasyOne Pro Respiratory Analysis System 2 Version history of the EasyOne Pro/LAB firmware... 2 Intended use of the EasyOne Pro Respiratory Analysis System... 3 Intended audience of this Operator’s Manual... 3 Using this Operator’s Manual... 3 Application Notes for further information... 3 Legal information... 4 Contact information... 4 Product registration... 4 Disposal... 4
2 Safety information
10 Classification... 10 General safety information... 10 Safety information regarding electromagnetic compatibility... 12 About requirements for connections to external devices... 13 List of applied parts... 14 List of equipment icons... 14
3 Introduction
15 About the two products EasyOne Pro and EasyOne Pro LAB... 15 Introduction to the EasyOne Pro Respiratory Analysis System... 16 Overview of EasyOne Pro Respiratory Analysis System... 16 About compatible printers and printer setup... 20 Connecting the components... 21 Turning EasyOne Pro/LAB on and off... 25 About using the touchscreen... 26 Overview of the user interface of EasyOne Pro/LAB... 27 List of terms and definitions... 29 List of tests and parameters... 30 List of abbreviations... 35 About sources for predicted normal values... 35
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
5/171
9
4 Cybersecurity
36 About cybersecurity... 36 About password policies and password expiration... 36 About periodical software updates and patches... 37 About backups... 37 Escalating in case of a security breach... 37 Dealing with a lost or stolen EasyOne Pro/LAB... 38 Using EasyOne Pro/LAB securely – general guidelines... 39
5 Connectivity and data exchange
40
About connectivity between EasyOne products... 40 About integration of EMR systems... 41 About supported EMR standards... 41 About possible workflows with your EMR system... 41 Overview of the integration process... 42 Overview of the plug-ins for EMR systems... 43 About the HL7 plug-in... 43 About the GDT plug-in... 43 About the XML file exchange plug-in... 44 About custom plug-ins... 44 Working with your EMR system... 45 Processing orders from your EMR system... 45 Initiating a spirometry test with the direct-start workflow... 46 Sending individual test results to your EMR system... 47
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
6/171
9
6 Performing lung function tests
48
Overview of the spirometry workflow... 48 About preparation of spirometry and instructions for the patient... 49 About quality messages for trials and quality grades for tests... 50 About posts and bronchodilation... 51 Performing basic spirometry without gas supply... 51 Performing a complete test... 51 Performing a bronchial provocation test... 58 Performing lung function tests with gas supply... 65 Mounting the motor and valve unit onto the flow sensor... 65 Dismount the motor and valve unit from the flow sensor... 66 Connecting the gas supply and opening the gas valves... 66 Contraindications for a DLCO test... 70 Performing a DLCO test... 70 Performing an MBW test... 77 Closing the gas valves and disconnecting the gas supply... 84 About interpreting results... 85 List of quality messages and quality grades... 86 About quality messages and quality grades... 86 List of quality messages for trials... 86 List of quality grades for tests... 92
7 Breathing maneuvers for all available tests
94
About breathing maneuvers for all available tests... 94 Performing the breathing maneuver for the FVC test... 94 Performing the breathing maneuver for the FVL test... 95 Performing the breathing maneuver for the MVV test... 96 Performing the breathing maneuver for the SVC test... 97
8 Hygiene and cleaning
98
Avoiding contamination while performing spirometry... 98 Cleaning instructions for EasyOne Pro/LAB... 99
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
7/171
9
9 Working with patient data and reports
100
Adding patient data to the database of EasyOne Pro/LAB... 100 Editing patient data with EasyOne Pro/LAB... 100 Merging two patient data records with EasyOne Pro/LAB... 101 Deleting patient data from the database of EasyOne Pro/LAB... 101 Viewing and printing reports with EasyOne Pro/LAB... 102 About the trend view with EasyOne Pro/LAB... 102 Assessing tests retrospectively and entering comments with EasyOne Pro/LAB ... 103 About exporting comprehensive data from EasyOne Pro/LAB... 104 About exporting XML from EasyOne Pro/LAB... 104
10 Configuration
105 About saving or discarding configuration changes of EasyOne Pro/LAB . . 105 About user handling and the default password... 105 Description of general configurations... 106 Utilities > Configuration > General > Header... 106 Utilities > Configuration > General > Storage... 106 Utilities > Configuration > General > System Settings... 107 Activating user handling and administering user accounts... 107 Description of test configurations for EasyOne Pro/LAB... 108 Utilities > Configuration > Test > General... 108 Utilities > Configuration > Test > Predicted... 109 Recalculating predicted values of previous test results... 113 Utilities > Configuration > Test > FVC / FVL... 114 Utilities > Configuration > Test > SVC... 115 Utilities > Configuration > Test > MVV... 115 Utilities > Configuration > Test > DLCO... 115 Utilities > Configuration > Test > FRC (MBW)... 116 Utilities > Configuration > Test > CalCheck... 117 Utilities > Configuration > Test > Provocation... 117 Loading and selecting a custom report layout of EasyOne Pro/LAB... 118 Description of printer configurations of EasyOne Pro/LAB... 118 Description of environment configurations... 119 Performing a software update... 120
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
8/171
9
11 Calibration check
122 Performing a calibration check... 122 Performing biological quality control... 124 About biological quality control... 124 Performing and assessing biological quality control... 125 List of quality criteria for biological quality control... 126 About a DLCO calibration check... 126 About an MBW calibration check... 126
12 Servicing and troubleshooting
127
Checking for correct operation of EasyOne Pro/LAB... 127 List of troubleshooting solutions... 127 About reactivating EasyOne Pro/LAB after storage... 128 Exporting logging information... 128 Creating screenshots... 129
13 Specifications and bibliography
130
List of specifications for EasyOne Pro... 130 List of specifications for EasyOne Pro LAB... 133 Description of test gas requirements... 136 List of order numbers and accessories for EasyOne Pro/LAB... 136 List of bibliographic references... 137
14 Index
138
A Appendix
143 List of used tools and frameworks in the EasyOne Pro/LAB software... 143 Microsoft Software License Agreement for Windows Embedded 8 Standard . . ... 144 GNU General Public License Version 3, 29 June 2007 for Sumatra PDF Reader . ... 156 Electromagnetic Compatibility (EMC)... 168 General... 168 Environment... 168 EMC conformance... 168 Safety information... 168 Compliant cables and accessories... 168 Wireless module... 168 Electromagnetic emission... 169 Electromagnetic immunity... 169
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
9/171
9
Safety information
2
Safety information
2.1
Classification In this manual, safety information is classified as follows: WARNING WARNING … … indicates a hazard. If not avoided, the hazard can result in death or serious injury. CAUTION CAUTION … … indicates a potential hazard. If not avoided, the hazard may result in minor injury and/or product/property damage.
2.2
General safety information Electric shock
To avoid risk of electric shock, this equipment must only be connected to a grounded mains supply. Do not position EasyOne Pro/LAB in a way that makes it difficult to operate the disconnection device.
Patient health hazard and false diagnosis Electric shock
Use only medical grade gases.
Patients and technicians may be exposed to dangerous voltage. To ensure electric safety connect only equipment, such as printers and networks, that complies with the IEC/EN 60950-1 standard and/or the IEC/ EN 60601-1 standard for electrical safety. Use only power cables approved by or acceptable to the authorities in your country.
Crosscontamination and damage to the environment
Fire
After use, the disposable spirette can be infectious or can be contaminated with allergens, irritants, or otherwise toxic substances. The used spirette can cause damage to the environment. Provide means for proper disposal of infectious waste, according to legal requirements. The plastic case can catch fire and cause burn injuries to the patient. The instrument is not suitable for use in the presence of flammable gases, such as anesthetic agents.
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
10/171
14
Safety information
Electric shock or hot surfaces Crosscontamination
Do not unscrew and open EasyOne Pro/LAB or any parts of it. Tuberculosis or other diseases spread by droplet nuclei and can cause aerosol infection. Proper attention to environmental engineering controls, such as ventilation, air filtration or ultraviolet decontamination of air, must be used.
Patient health hazard and device malfunction
For spirometry tests with gas supply, off-specification test gas can cause adverse reaction, poisoning, allergic reaction, or other harm to the patient. Also, off-specification test gas can cause device malfunction. Use only appropriate gas, according to the test gas requirements. Description of test gas requirements, 136
False diagnosis
For unforeseen reasons, malfunction of EasyOne Pro/LAB can lead to false results and false diagnosis. Perform calibration checks periodically. Calibration check, 122 Checking for correct operation of EasyOne Pro/LAB, 127
False diagnosis
EasyOne Pro/LAB can be damaged when dropped or during transport. Do an incoming inspection when you first receive EasyOne Pro/LAB. If you detect a damage, contact your EasyOne dealer or the ndd Servicing Department. Do not drop EasyOne Pro/LAB. If EasyOne Pro/LAB has been dropped, check for correct operation of EasyOne Pro/LAB. Checking for correct operation of EasyOne Pro/LAB, 127
Patient health hazard and false diagnosis
Inadequate qualification of the personnel can put the patient’s health at risk and can result in incorrect execution of tests, false results, and false diagnosis and/or high costs. EasyOne Pro/LAB must only be used by qualified personnel. Intended audience of this Operator’s Manual, 3
False diagnosis
Setup with wrong environment data can cause false results and false diagnosis. Verify the set up environment data. Description of environment configurations, 119
Failed update and data loss
An update may fail. For various other reasons, data loss can occur and is generally unforeseen. Perform backups of the database of EasyOne Pro/LAB frequently. About exporting comprehensive data from EasyOne Pro/LAB, 104
Malfunction
Non-original accessories, like tubes or cables can cause malfunction. Only use original accessories by ndd. List of order numbers and accessories for EasyOne Pro/LAB, 136
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
11/171
14
Safety information
False diagnosis
Non-original respiratory tubes can cause measurement error and false results. You must only use spirette respiratory tubes by the manufacturer ndd to assure accuracy, long-life, and full warranty coverage. List of order numbers and accessories for EasyOne Pro/LAB, 136
Network connection
Connection of EasyOne Pro/LAB to an IT network that includes other equipment could result in previously unidentified risks to patients, operators, or third parties. Subsequent changes to the IT network could introduce new risks and require additional analysis. Changes to the IT network include: • changes in the IT network configuration, • connection of additional items to the IT network, • disconnecting items from the IT network, • update of equipment connected to the IT network, and • upgrade of equipment connected to the IT network. It is your responsibility to identify, analyze, evaluate, and control these risks. IEC 80001-1:2010 provides guidance to address these risks.
Patient health hazard and false diagnosis
2.3
EasyOne Pro/LAB measures and presents data reflecting a patient’s physiological condition. When reviewed by a qualified physician or clinician, this data can be useful in determining a diagnosis. However, the data should not be used as sole means for determining a patient’s diagnosis.
Safety information regarding electromagnetic compatibility Influence by HF surgical equipment
Electrical devices with a high RF power output during intended use (e.g. High frequency (HF) surgical equipment) should not be operated in parallel with EasyOne Pro/LAB.
Portable wireless communications equipment
Portable wireless communications equipment such as wireless home network devices, mobile phones, cordless telephones and their base stations, walkietalkies, etc. can affect EasyOne Pro/LAB. Keep a distance of at least 30 cm (12 inches) to any part of EasyOne Pro/LAB.
Increased emissions or decreased immunity
Accessories, transducers, and cables other than those specified by the manufacturer or replacement parts for internal components may result in increased emissions or decreased immunity of EasyOne Pro/LAB.
Proximity to other equipment
EasyOne Pro/LAB should not be used adjacent to or stacked with other equipment. Should close proximity or stacking be unavoidable, however, the configuration in which it is used needs to be watched closely to ensure that the equipment is functioning normally. See Appendix
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
12/171
14
Safety information
2.4
About requirements for connections to external devices In your capacity as the operator of the medical electrical device you are obliged to ensure that the specific, applicable safety requirements for the operation of a medical-electrical device are complied with. The following conditions must be met: • All equipment operated in the patient environment must meet the requirements of IEC 60601-1. • All equipment set up outside the patient environment must meet the requirements of the applicable IEC or ISO safety standards (e.g. IEC 60950-1).
Patient environment
If devices that do not fulfill the requirements of IEC standard 60601-1 are operated in the patient environment, it must be ensured that the maximum allowed touch currents will not be exceeded. The following limits are applicable: • normal condition: 100 μA • with interruption of the (not permanently connected) protective earth conductor: 500 μA Appropriate measures must be taken if these limits are exceeded. Suggestions: • additional protective earth connection of the PC or • isolating transformer for the PC or • isolating transformer with built-in power outlet strip for the PC and the devices connected to it EN 60601-1:2006 specifies the requirements for power outlet strips. Bear in mind that the touch currents may vary with the system configuration.
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
13/171
14
Safety information
2.5
List of applied parts Applied parts are those parts of medical electrical equipment that necessarily come into physical contact with the patient to perform the intended use of the device. The following part of EasyOne Pro/LAB is an applied part: • Flow sensor Overview of EasyOne Pro Respiratory Analysis System, 16
2.6
List of equipment icons Read usage instructions.
Caution
Follow instructions for use.
Do not reuse (single-patient use).
CE marked per the Medical Device Directive 93/42/EEC.
Product certification for the USA and Canada. ®
Date of manufacture-the number found under this icon is the date of manufacture. Manufacturer
Caution: Federal law restricts this device to sale by or on the order of a licensed healthcare practitioner. In the European Union, the product you have purchased should not be disposed of as unsorted municipal waste. Please make use of your local WEEE collection facilities to dispose of this product and otherwise observe all applicable requirements. Instrument classification: Type BF applied part
Universal Serial Bus (USB)-standard for connecting devices and data transfer, applies to cables and connectors
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
14/171
14
Introduction
3
Introduction
3.1
About the two products EasyOne Pro and EasyOne Pro LAB In the product family EasyOne Pro Respiratory Analysis System, there are two products, EasyOne Pro and EasyOne Pro LAB. EasyOne Pro LAB has the same functionality as EasyOne Pro, but in addition to that can perform the multibreath washout test (MBW test) to determine the functional residual capacity (FRC). This Operator’s Manual is for both products, EasyOne Pro and EasyOne Pro LAB. Therefore, both products are commonly referred to as EasyOne Pro/LAB in this Operator’s Manual.
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
15/171
35
Introduction
3.2
Introduction to the EasyOne Pro Respiratory Analysis System
3.2.1
Overview of EasyOne Pro Respiratory Analysis System The EasyOne Pro/LAB respiratory analysis system A
B
C
D
E
F
G
H
A Patient tube
B Flow sensor cable
C Flow sensor
D Motor and valve unit
E
F
spirette respiratory tube
G Power button
Flow sensor holder
H Touchscreen
The EasyOne Pro Respiratory Analysis System
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
16/171
35
Introduction I
M I
J
N
O
P
K
Q
L
R
S
2x USB ports
T
U
V
W
J
2x network ports (Ethernet)
K Slot for USB flash drive
L
Slot for inserting the flow sensor holder
M Monitor port (HDMI)
N Monitor port (VGA/D-Sub)
O Connector for the temperature and humidity sensor
P Filter pack
Q Port for the DLCO gas tube
R Potential equalization pin
S Power port
T
U Port for the O2 gas tube (for MBW tests, EasyOne Pro LAB only)
V Connector for the flow sensor cable
Mains switch
W Connector for the patient tube Ports and connectors of EasyOne Pro/LAB
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
17/171
35
Introduction
The one-way valve for multi-patient use The one-way valve ensures that DLCO gas or O2 gas only flows in one direction. The one-way valve can be used for multiple patients and must be replaced once a year. The one-way valve is designed to work accurately with EasyOne Pro/LAB. For reliable results, use only the original one-way valve manufactured by ndd. The figure shows how to insert the one-way valve.
Attaching the one-way valve
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
18/171
35
Introduction
The DLCO barriette and FRC barriette for single-patient use The DLCO barriette and the FRC barriette connect the spirette respiratory tube to gas system. To ensure hygienic testing, the DLCO barriette and the FRC barriette are disposables for single-patient use. The DLCO barriette and the FRC barriette are designed to work accurately with EasyOne Pro/LAB. For reliable results, use only the original DLCO barriette and the original FRC barriette manufactured by ndd. The figures show how to insert the DLCO barriette and the FRC barriette.
Inserting the DLCO barriette
Inserting the FRC barriette
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
19/171
35
Introduction
The spirette respiratory tube for single-patient use
The spirette respiratory tube
To ensure hygienic testing, the spirette respiratory tube is a disposable for single-patient use. The spirette is designed to work accurately with the ultrasonic sensor of EasyOne Pro/LAB. For reliable results, use only the original ndd spirette. CAUTION FALSE DIAGNOSIS Non-original respiratory tubes can cause measurement error and false results. You must only use spirette respiratory tubes by the manufacturer ndd to assure accuracy, long-life, and full warranty coverage. List of order numbers and accessories for EasyOne Pro/LAB, 136
3.2.2
About compatible printers and printer setup Printers can be connected directly via USB, or printers can be shared and accessed over the network. Refer to the Application Notes below for recommended printers and printer setup. Application Note Recommended Printers for EasyOne Pro Application Note Using Printers with EasyOne Pro Application Notes for further information, 3
Operator’s Manual V5.0 • EasyOne Pro/LAB Rev. 05 © ndd Medizintechnik AG • Technoparkstrasse 1, 8005 Zurich, Switzerland • www.ndd.ch
20/171
35