Use Guide
132 Pages
Preview
Page 1
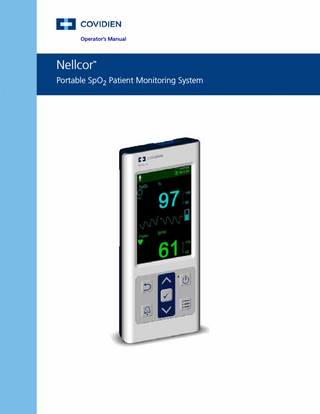
Operator’s Manual
Nellcor
TM
Portable SpO2 Patient Monitoring System
COVIDIEN, COVIDIEN with logo, the Covidien logo and positive results for life are U.S. and internationally registered trademarks of Covidien AG. Other brands are trademarks of a Covidien company. ©2014 Covidien. All rights reserved. Microsoft and Windows CE are registered trademarks of Microsoft Corporation in the United States and other countries. The information contained in this manual is the sole property of Covidien and may not be duplicated without permission. This manual may be revised or replaced by Covidien at any time and without notice. It is the responsibility of the reader to have the most current applicable version of this manual. If in doubt, contact Covidien Technical Services. While the information set forth herein is believed to be accurate, it is not a substitute for the exercise of professional judgment. The equipment and software should only be operated and serviced by trained professionals. Covidien’s sole responsibility with respect to the equipment and software, and its use, is as stated in the limited warranty provided. Nothing in this manual shall limit or restrict in any way Covidien’s right to revise or otherwise change or modify the equipment and software described herein, without notice. In the absence of an express, written agreement to the contrary, Covidien has no obligation to furnish any such revisions, changes, or modifications to the owner or user of the equipment and software described herein.
Table of Contents 1 Introduction
...1-1
1.1 Overview...1-1 1.2 Safety Information...1-1 1.2.1 Safety Symbols...1-1 1.2.2 Explosion, Shock, and Toxicity Hazards...1-2 1.2.3 Patient Monitoring and Safety...1-2 1.2.4 Monitoring System Operation and Service...1-3 1.2.5 Monitoring System Readings...1-4 1.2.6 Sensors, Cables, and Other Accessories...1-5 1.2.7 Electromagnetic Interference...1-6 1.2.8 Connections with Other Equipment...1-7 1.2.9 Monitoring System Storage, Transport, and Disposal ...1-7
1.3 Obtaining Technical Assistance
...1-8
1.3.1 Technical Services...1-8 1.3.2 Related Documents...1-9
1.4 Revision History...1-9 1.5 Warranty Information ...1-9 2 Product Overview
...2-1
2.1 Overview...2-1 2.2 Product Description ...2-1 2.3 Intended Use...2-2 2.4 Product Views...2-3 2.4.1 Front Panel and Display Components...2-3 2.4.2 Rear Panel...2-9 2.4.3 Product and Carton Label Symbols...2-9
3 Installation
...3-1
3.1 Overview...3-1 3.2 Unpacking and Inspection...3-1 3.3 Setup...3-2 3.3.1 Using the Batteries...3-2 3.3.2 Connecting a Nellcor™ Pulse Oximetry Sensor...3-3
4 Operation
...4-1
4.1 Overview
...4-1
iii
4.2 Operation Basics
...4-1
4.2.1 Power On the Monitoring System...4-1 4.2.2 Power Off the Monitoring System...4-3 4.2.3 Navigate the Menus...4-4
4.3 Menu Structure and Factory Defaults...4-4 4.4 Patient Monitoring...4-6 4.4.1 Set Patient Mode...4-6 4.4.2 Save a Spot Reading...4-7
4.5 Alarms and Alarm Limits Management
...4-8
4.5.1 Alarm Indicators...4-8 4.5.2 Pausing an Audible Alarm...4-10 4.5.3 Adjusting Alarm Limits...4-11 4.5.4 Using the SatSeconds™ Alarm Management System...4-14
4.6 Additional Patient Modes
...4-16
4.6.1 Set Response Mode...4-16 4.6.2 Set Homecare Mode...4-17 4.6.3 Set Sleep Study Mode...4-21
4.7 Adjust Brightness and Volume
...4-24
4.7.1 Adjust Brightness ...4-24 4.7.2 Adjust Volume...4-25 4.7.3 Screen Saver...4-27
4.8 Service Menu...4-27 4.9 Maintenance Reminder...4-28 5 Data Management
...5-1
5.1 Overview...5-1 5.2 Monitoring History...5-1 5.3 External Data Communication...5-4 5.3.1 Monitoring History (Trend Data) Download...5-5 5.3.2 Firmware Upgrades...5-15
6 Performance Considerations
...6-1
6.1 Overview...6-1 6.2 Oximetry Considerations...6-1 6.2.1 Pulse Rates 6.2.2 Saturation
...6-1 ...6-1
6.3 Performance Considerations
...6-2
6.3.1 Overview...6-2 6.3.2 Patient Conditions...6-2 6.3.3 Sensor Performance Considerations ...6-3 6.3.4 Reducing EMI (Electromagnetic Interference) ...6-4
iv
7 Preventive Maintenance
...7-1
7.1 Overview...7-1 7.2 Cleaning...7-1 7.3 Recycling and Disposal...7-2 7.4 Battery Maintenance...7-2 7.5 Periodic Safety Checks...7-3 7.6 Service...7-3 8 Troubleshooting
...8-1
8.1 Overview...8-1 8.2 General...8-1 8.3 Error Conditions...8-2 8.4 Return...8-4 9 Accessories
...9-1
9.1 Overview...9-1 9.2 Nellcor™ Pulse Oximetry Sensors...9-1 9.2.1 Nellcor™ Sensor Features...9-3 9.2.2 Biocompatibility Testing...9-3
9.3 Optional Equipment
...9-3
10 Theory of Operations
...10-1
10.1 Overview...10-1 10.2 Theoretical Principles...10-1 10.3 Automatic Calibration...10-2 10.4 Functional Testers and Patient Simulators...10-2 10.5 Unique Technologies...10-3 10.5.1 Functional versus Fractional Saturation...10-3 10.5.2 Measured versus Calculated Saturation...10-4 10.5.3 Data Update Period, Data Averaging, and Signal Processing...10-4
10.6 System Features
...10-5
10.6.1 Nellcor™ Sensor Technology...10-5 10.6.2 SatSeconds™ Alarm Management Parameter...10-6
11 Product Specifications
...11-1
11.1 Overview...11-1 11.2 Physical Characteristics...11-1
v
11.3 Electrical ...11-2 11.4 Environmental Conditions...11-2 11.5 Tone Definition...11-3 11.6 Sensor Accuracy and Ranges...11-4 11.7 Sound Pressure...11-7 11.8 Product Compliance...11-7 11.9 Manufacturer’s Declaration...11-8 11.9.1 Electromagnetic Compatibility (EMC)...11-8 11.9.2 Sensor and Cable Compliance...11-12 11.9.3 Safety Tests...11-14
11.10 Essential Performance A Clinical Studies
...11-14
... A-1
A.1 Overview... A-1 A.2 Methods... A-1 A.3 Study Population... A-2 A.4 Study Results... A-2 A.5 Adverse Events or Deviations ... A-3 A.6 Conclusion... A-4
vi
List of Tables Table 1-1. Table 2-1. Table 2-2. Table 3-1. Table 4-1. Table 4-2. Table 5-1. Table 8-1. Table 9-1. Table 11-1. Table 11-2. Table 11-3. Table 11-4. Table 11-5. Table 11-6. Table 11-7. Table 11-8. Table 11-9. Table 11-10. Table 11-11. Table 11-12. Table A-1. Table A-2.
Safety Symbol Definitions... 1-1 Display Colors ... 2-8 Symbol Descriptors... 2-9 Standard Items ... 3-1 Menu Structure and Available Options ... 4-4 Alarm Conditions ... 4-9 Monitoring Status Codes ... 5-4 Common Problems and Resolutions ... 8-2 Nellcor™ Sensor Models and Patient Sizes... 9-1 Transport, Storage, and Operating Condition Ranges ... 11-2 Tone Definitions... 11-3 Trends ... 11-4 Pulse Oximetry Sensor Accuracy and Ranges ... 11-5 Sound Pressure in Decibels ... 11-7 Electromagnetic Emissions Guidelines and Compliance ... 11-9 Electromagnetic Immunity Guidelines and Compliance... 11-10 Recommended Separation Distance Calculations ... 11-11 Recommended Separation Distances... 11-12 Sensor and Cable Length ... 11-13 Enclosure Leakage Current Specification ... 11-14 Patient Leakage Current Values... 11-14 Demographic Data ...A-2 SpO2 Accuracy for Nellcor™ Sensors vs. CO-oximeters ...A-2
vii
Page Left Intentionally Blank
viii
List of Figures Figure 2-1. Figure 2-2. Figure 2-3. Figure 3-1. Figure 3-2. Figure 3-3. Figure 4-1. Figure 4-2. Figure 4-3. Figure 4-4. Figure 4-5. Figure 4-6. Figure 4-7. Figure 4-8. Figure 4-9. Figure 4-10. Figure 4-11. Figure 4-12. Figure 4-13. Figure 4-14. Figure 4-15. Figure 4-16. Figure 4-17. Figure 4-18. Figure 4-19. Figure 4-20. Figure 4-21. Figure 4-22. Figure 5-1. Figure 5-2. Figure 5-3. Figure 5-4. Figure 5-5. Figure 5-6. Figure 5-7. Figure 5-8. Figure 5-9. Figure 5-10. Figure 5-11. Figure 5-12. Figure 7-1.
Front Panel Components ... 2-3 Display Components ... 2-5 Rear Panel Components ... 2-9 Sensor Port Cover ... 3-4 Interface Cable (DEC-4) or Sensor Cable Connection ... 3-4 Interface Cable (Optional) Connection to Sensor ... 3-4 Example Initial Screen ... 4-2 Main Monitoring Screen ... 4-3 Patient Mode Menu ... 4-7 Save Spot Reading ... 4-8 Main Monitoring Screen ...4-12 Alarm Limits Menu ...4-13 High SpO2 Setting ...4-14 SatSeconds Setting ...4-15 Response Mode Menu ...4-16 Patient Mode Menu Item ...4-17 Homecare Mode Menu Item ...4-18 Pass Code Entry for Homecare Mode ...4-19 Prompt to Delete or Keep Monitoring History ...4-20 Homecare Mode Monitoring Screen ...4-20 Patient Mode Menu Item ...4-21 Sleep Study Mode Menu Item ...4-22 Pass Code Entry for Sleep Mode ...4-23 Sleep Study Mode ...4-23 Device Settings Menu ...4-24 Brightness Setting Menu ...4-25 Sound Settings Menu ...4-26 Example Volume Setting ...4-27 Monitoring History Menu ... 5-2 Monitoring History Screen ... 5-2 Continuous Data Screen (Interval 100) and Scroll Bar ... 5-3 Transfer Data Type ... 5-7 Transfer Data by USB ... 5-8 Sample Trend Data Printout ... 5-9 Bridge Driver Installer window ...5-10 New Hardware Wizard Screen ...5-11 Device Manager Button Under Hardware Tab ...5-12 Hardware list in Device Manager window ...5-13 Sample Initial USB to UART Bridge Properties Window ...5-14 Baud rate list under Port Settings tab ...5-15 Monitoring System Cleaning ... 7-2
ix
Figure 9-1. Figure 9-2. Figure 9-3. Figure 9-4. Figure 10-1. Figure 10-2. Figure 10-3. Figure 10-4. Figure 10-5. Figure A-1.
x
Standard Protective Covers ... 9-3 Transport Protective Cover ... 9-4 Carrying Case ... 9-5 Extension Cable (DEC-4) ... 9-5 Oxyhemoglobin Dissociation Curve ...10-4 Series of SpO2 Events ...10-6 First SpO2 Event: No SatSeconds Alarm ...10-7 Second SpO2 Event: No SatSeconds Alarm ...10-8 Third SpO2 Event: Triggers SatSeconds Alarm ...10-9 Modified Bland-Altman Plot ...A-3
1 Introduction
1.1
Overview This manual contains information for operating the Nellcor™ Portable SpO2 Patient Monitoring System. Before operating the monitoring system, thoroughly read this manual. This manual applies to the following product: PM10N
1.2
Note: Before use, carefully read this manual, the Instructions for Use for the accessories, and all precautionary information and specifications.
Safety Information This section contains important safety information related to general use of the Nellcor™ Portable SpO2 Patient Monitoring System. Other important safety information appears throughout the manual. The Nellcor™ Portable SpO2 Patient Monitoring System is referred to as the “monitoring system” throughout this manual.
1.2.1 Safety Symbols
Table 1-1. Safety Symbol Definitions Symbol
Definition WARNING Alerts users to potential serious outcomes (death, injury, or adverse events) to the patient, user, or environment.
1-1
Introduction
Table 1-1. Safety Symbol Definitions Symbol
Definition Caution Identifies conditions or practices that could result in damage to the equipment or other property. Note Provides additional guidelines or information.
1.2.2 Explosion, Shock, and Toxicity Hazards
WARNING: Explosion hazard - Do not use the monitoring system in the presence of flammable anesthetics. WARNING: Shock hazard-Do not pour or spill liquids onto the monitoring system. WARNING: Shock hazard-Firmly close the battery cover to prevent moisture from entering the monitoring system. WARNING: The LCD panel (display) contains toxic chemicals. Do not touch broken LCD panels. Physical contact with a broken LCD panel can result in transmission or ingestion of toxic substances.
1.2.3 Patient Monitoring and Safety
1-2
WARNING: Always disconnect and remove the monitoring system and sensors during magnetic resonance imaging (MRI) scanning. Attempting to use the monitoring system during an MRI procedure could cause burns or adversely affect the MRI image or the monitoring system's accuracy.
Operator’s Manual
Safety Information
WARNING: Keep patients under close surveillance when monitoring. It is possible, although unlikely, that radiated electromagnetic signals from sources external to the patient and the monitoring system can cause inaccurate measurement readings. WARNING: As with all medical equipment, carefully route patient cabling to reduce the possibility of patient entanglement or strangulation. WARNING: Do not lift or carry the monitoring system by the pulse oximetry sensor or pulse oximetry interface cable. The cable may disconnect and cause the monitoring system to drop on a patient or cause damage to monitoring system surfaces.
1.2.4 Monitoring System Operation and Service
WARNING: Inspect the monitoring system and all accessories before use to ensure there are no signs of physical damage or improper function. Do not use if damaged. WARNING: To ensure accurate performance and prevent device failure, do not expose the monitoring system to extreme moisture, such as direct exposure to rain. Such exposure may cause inaccurate performance or device failure. Do not immerse in water, solvents, or cleaning solutions, since the monitoring system and pulse oximetry sensors and connectors are not waterproof. WARNING: Do not sterilize the monitoring system by irradiation, steam, or ethylene oxide. WARNING: The monitoring system should not be used adjacent to or stacked with other equipment. If adjacent or stacked use is necessary, observe the monitoring system to verify normal operation in the desired configuration.
Operator’s Manual
1-3
Introduction
WARNING: The only user-serviceable parts inside the monitoring system are the four AA batteries. While users can open the battery cover to change the batteries, only qualified service personnel should remove the cover or access internal components for any other reason. Users should not modify any components of the monitoring system. WARNING: Do not spray, pour, or spill any liquid on the monitoring system, its accessories, connectors, switches, or openings in the casing, since this may cause damage to the monitoring system. Never place fluids on the monitoring system. If fluid spills on the monitoring system, remove batteries, wipe all components dry immediately, and have the monitoring system serviced to ensure no hazard exists. WARNING: Do not damage the batteries by applying pressure. Do not throw, hit, or drop or impact the batteries. WARNING: Keep the monitoring system and batteries out of reach of children to avoid any accidents. Caution: The monitoring system may not operate properly if it is operated or stored at conditions outside the ranges stated in this manual, or if it is subjected to excessive shock or dropping.
1.2.5 Monitoring System Readings
1-4
WARNING: The monitoring system may remain attached to the patient during defibrillation or during use of an electrosurgical unit; however, the monitoring system is not defibrillator-proof, and readings may be inaccurate during defibrillation and shortly thereafter.
Operator’s Manual
Safety Information
WARNING: Check the patient's vital signs by alternate means should there be any doubt about the accuracy of any measurement. Request a qualified service technician confirm the monitoring system is functioning correctly. WARNING: For best product performance and measurement accuracy, use only accessories supplied or recommended by Covidien. Use accessories according to their respective Instructions for Use.
1.2.6 Sensors, Cables, and Other Accessories
WARNING: Before use, carefully read the pulse oximetry sensor Instructions for Use, including all warnings, cautions, and instructions. WARNING: Use only the Covidien-approved pulse oximetry sensors, interface cables, and accessories. Use of other sensors, cables, and accessories can result in inaccurate readings and increased monitoring system emissions. WARNING: Do not use any other cables to extend the length of the Covidien-approved interface cable. Increasing the length will degrade signal quality and may lead to inaccurate measurements. WARNING: To prevent damage, avoid undue bending of the sensor cable. WARNING: The sensor disconnect error message and associated alarm indicate the pulse oximetry sensor is either disconnected or has faulty wiring. Check the connection and, if necessary, replace the sensor, the pulse oximetry cable, or both.
Operator’s Manual
1-5
Introduction
1.2.7 Electromagnetic Interference
1-6
WARNING: Any radio frequency transmitting equipment or other nearby sources of electrical noise may result in disruption of the monitoring system. WARNING: The monitoring system is designed for use in environments in which the signal can be obscured by electromagnetic interference. During such interference, measurements may seem inappropriate or the monitoring system may not seem to operate correctly. WARNING: Large equipment using a switching relay for its power on/off may affect monitoring system operation. Do not operate the monitoring system in such environments. Caution: This device has been tested and found to comply with the limits for medical devices related to IEC 60601-1-2: 2007. These limits are designed to provide reasonable protection against harmful interference in a typical medical installation. Caution: This monitoring system generates, uses, and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to other devices in the vicinity. If interference is suspected, move pulse oximetry cables away from the susceptible device. Caution: Be aware of possible interference from sources of electromagnetic interference, such as cellular phones, radio transmitters, motors, telephones, lamps, electrosurgical units, defibrillators, and other medical devices. If pulse oximetry readings are not as expected for the patient’s condition, remove the sources of possible interference.
Operator’s Manual
Safety Information
1.2.8 Connections with Other Equipment
Caution: Accessory equipment connected to the monitoring system's data interface must be certified according to IEC Standard 60950-1 for data-processing equipment. All combinations of equipment must be in compliance with IEC Standard 60601-1:2005 Requirements for Medical Electrical Systems. Anyone who connects additional equipment to the signal input or signal output port configures a medical system and is therefore responsible for ensuring the system complies with the requirements of IEC Standard 60601-1:2005 and IEC Standard 60601-1-2:2007. Caution: When connecting the monitoring system to any instrument, verify proper operation before clinical use. Caution: Anyone who connects a PC to the data output port configures a medical system and is therefore responsible for ensuring that the system complies with the requirements of IEC Standard 60601-1-1 and the electromagnetic compatibility IEC Standard 60601-1-2.
1.2.9 Monitoring System Storage, Transport, and Disposal
Caution: Remove the batteries from the monitoring system before placing it in storage or when not using it for a long period. Caution: Do not short-circuit the batteries, as they may generate heat. To avoid shortcircuiting, do not let the batteries come in contact with metal objects at any time, especially during transport. Caution: Follow local government ordinances and recycling instructions regarding disposal or recycling of the monitoring system and its components, including batteries and accessories.
Operator’s Manual
1-7
Introduction
1.3
Obtaining Technical Assistance
1.3.1 Technical Services
For technical information and assistance, contact Covidien or a local Covidien representative. Covidien Technical Services: Patient Monitoring 15 Hampshire Street Mansfield, MA 02048 USA 1.800.635.5267, 1.925.463.4635, or contact a local Covidien representative www.covidien.com
When calling Covidien or a local Covidien representative, have the monitoring system serial number available. Provide the firmware version number listed at power-on self-test (POST).
1-8
Operator’s Manual
Revision History
1.3.2 Related Documents
Nellcor™ Portable SpO2 Patient Monitoring System Home Use Guide - Provides basic information for operating the monitoring system, handling alarms, and troubleshooting errors or malfunctions. This manual is directed to home caregivers. Nellcor™ Pulse Oximetry Sensor Instructions for Use - Guides sensor selection and usage. Before attaching any of the various Covidien-approved pulse oximetry sensors to the monitoring system, refer to the individual Instructions for Use. Saturation Accuracy Grid - Provides sensor-specific guidance related to desired SpO2 saturation accuracy measurements. Available online at www.covidien.com. Nellcor™ Portable SpO2 Patient Monitoring System Service Manual - Provides information to qualified service technicians for use when servicing the monitoring system. 1.4
Revision History The documentation part number and revision number indicate its current edition. The revision number changes when Covidien prints a new edition. Minor corrections and updates incorporated at reprint do not cause a change in the revision number. Extensive changes may require a new document part number.
1.5
Warranty Information The information contained in this document is subject to change without notice. Covidien makes no warranty of any kind with regard to this material, including, but not limited to, the implied warranties or merchantability and fitness for a particular purpose. Covidien shall not be liable for errors contained herein or for incidental or consequential damages in connection with the furnishing, performance, or use of this material.
Operator’s Manual
1-9
Introduction
Page Left Intentionally Blank
1-10
Operator’s Manual