Ohio Medical Corporation
Push-To-Set Intermittent Suction Unit Instructions for Use Rev 9 March 2013
Instructions for Use
14 Pages
Preview
Page 1
Push-To-SetTM Intermittent Suction Unit (PTS-ISU) Instructions for Use (Adult/Pediatric/Neonatal)
Low Vacuum mmHg
Low Vacuum mmHg
®
®
®
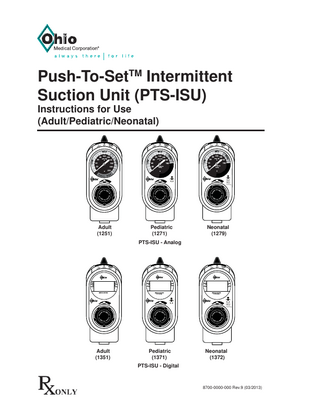
Adult (1251)
Pediatric (1271)
Neonatal (1279)
PTS-ISU - Analog
®
F H
F H
M
M
L
L
H
H
H
M
M
M
M
L
L
L
Adult (1351)
L
MEDICAL VACUUM 0-160 mmHg
MEDICAL VACUUM
®
®
H
®
Pediatric (1371)
MEDICAL VACUUM 0-100 mmHg
®
Neonatal (1372)
PTS-ISU - Digital
8700-0000-000 Rev.9 (03/2013)
Table of Contents Safety Instructions ... 2 Intended Use ... 2 Receiving/Inspection... 2 User Responsibility ... 2 Definitions ... 3 Regulator Identification ... 4 Operation... 5 Cleaning and Disinfection ... 9 Troubleshooting ... 9 Timing Cycle Adjustment... 10 Adapter/Probe Installation ... 12 Warranty ... 13 Safety Instructions This manual provides you with important information about the Push-To-SetTM Intermittent Suction Unit (PTS-ISU). To ensure the safe and proper use of this device, READ and UNDERSTAND all of the safety and operating instructions. IF YOU DO NOT UNDERSTAND THESE INSTRUCTIONS, OR HAVE ANY QUESTIONS, REFER TO SERVICE MANUAL, CONTACT YOUR SUPERVISOR, DEALER OR THE MANUFACTURER BEFORE ATTEMPTING TO USE THE DEVICE. Intended Use: The vacuum regulator is intended to be used in the medical facility as a means to evacuate media (i.e. fluids) from the body. DO NOT use this vacuum regulator for anything other than its intended use. Receiving / Inspection: Remove product from package and inspect for damage. If product is damaged, DO NOT USE and contact your dealer or equipment provider. User Responsibility This Product will perform as described in this operating manual and accompanying labels and/or inserts, when assembled, operated, maintained and repaired in accordance with the instructions provided. This Product must be checked periodically. A defective product should not be used. Parts that are broken, missing, worn, distorted or contaminated should be replaced immediately. Should such repair or replacement become necessary, see the Ohio Medical service manual for service or repairs to this product. For service advice, Ohio Medical recommends that a telephone request be made to the nearest Ohio Medical Regional Service Center. This product and any of its parts should only be repaired using written instructions provided by Ohio Medical or by Ohio Medical trained personnel. The Product must not be altered without the prior written approval of Ohio Medical’s Quality Assurance Department. The user of this Product shall have the sole responsibility for any malfunction which results from improper use, faulty maintenance, improper repair, damage, or alteration by anyone other than Ohio Medical. AAA A 12345
2
This alpha character indicates the year of product manufacture and when the serial number was assigned; “L” = 2007, “M” = 2008, “N” = 2009, etc. “I” and “O” are not used.
8700-0000-000 Rev.9 (03/2013)
2
Definitions WARNING CAUTION Note Important |O|O (INT) | (CONT) O (OFF) High Flow Low Vacuum High Flow High Vacuum
= possible injury to patient or operator = possible damage to equipment = Provides additional information to clarify a point in the text. = Similar to a note but of greater emphasis = Attention. Alerts you to a warning or caution in the text. = intermittent; cycles ON and OFF = continuous, ON = OFF Consult Instructions for Use
= high flow, low vacuum = high flow, high vacuum
Abbreviations in ISU kPa mmHg mm mL °C °F
Inch Intermittent Suction Unit Kilo pascals (kPa x 7.50 = 1 mmHg) Millimeters of mercury (mmHg x 0.133 = kPa) Millimeters Milliliters Degrees Celsius Degrees Fahrenheit
N-m ft-lb in-lb DISS NPTF NEO PTS PTFE
Newton-Meter (N-m x 0.737 = ft-lb) Foot-Pound Force (ft-lb x 1.356 = N-m) Inch-Pound Force (ft-lb x 12 = in-lb) Diameter Index Safety System National Pipe Thread Female (USA) Neonatal Push-To-Set™ Polytetrafluoroethylene
CAUTIONS Only competent individuals trained in the repair of this equipment should attempt to service it. Detailed information for more extensive repairs is included in the service manual for users having proper knowledge, tools and test equipment, and for service representatives trained by Ohio Medical. Not for field or transport use.* * The categories of Field and Transport Use are specifically defined in ISO 10079-3 “Field” means accidents or emergencies outside the hospital. “Transport” means use in ambulances, cars and airplanes. These situations may expose the equipment to uneven support, dirt, water, mechanical shock and temperature extremes. Ohio Medical suction equipment has not been tested to comply with the specific requirements of these categories.
Important: BATTERY LOW INDICATOR: When a battery icon appears on the gauge it indicates that the battery is low. Please take the unit out of service immediately and contact an Ohio Medical Customer Service Representative for battery replacement. NOTE: If the low battery condition is not addressed and the battery becomes fully depleted, the gauge will not show any readout, including the low battery icon or gauge pressure. If the gauge were to go blank during suctioning, the unit will continue to suction and the intermittent feature will continue to operate. Once completing that procedure, it is important to immediately take the unit out of service and contact an Ohio Medical Customer Service Representative for battery replacement. 3
8700-0000-000 Rev.9 (03/2013)
3
Regulator Identification Push-To-SetTM Intermittent Suction Unit (PTS-ISU) 1
Figure 1 Vacuum Gauge/Analog Fitting/Patient Port (inlet) Mode Selector Switch Probe/Adapter Port (outlet) Suction Control Knob Vacuum Gauge/Digital (Push-To-Set™)
®
F H
F H
M
M
L
L
MEDICAL VACUUM
®
The pediatric and neonatal models are identified by the Baby Icon on the front right when looking at the unit.
4
8700-0000-000 Rev.9 (03/2013)
4
Operation WARNINGS
This device is to be used only by persons who have been adequately instructed in its use.
Do not use this device in the presence of flammable anesthetics. Static charges may not dissipate and a possible explosion hazard exists in the presence of these agents.
Failure to follow these instructions may cause damage to the vacuum regulator.
Factory settings may be impacted during transport therefore, the unit’s timing cycle MUST be checked prior to use and adjusted if necessary (see Troubleshooting: Timing Cycle Adjustment)
Equipment Setup Insert the adapter/probe into the vacuum wall outlet. If the regulator is mounted elsewhere, connect a vacuum supply hose between the regulator’s adapter/probe and the wall outlet. WARNING
Connection to positive pressure sources such as oxygen and medical air, even momentarily, could injure the patient or operator.
CAUTION
Connection to positive pressure sources such as oxygen and medical air, even momentarily, could damage the equipment.
Use hospital-supplied suction tubing between the vacuum regulator and the collection container, as well as between the patient port of the collection container and the patient (minimum inside diameter is 6 mm [0.25 in]). An Ohio Medical high flow suction filter and/or overflow safety trap (OST) should be used between the collection container and regulator to prevent contamination of the regulator, wall outlet and pipeline system. ISO 10079-3 (Section 5.1.2) states that “the usable volume of the collection container shall not be less than 500 mL.” High Flow Suction Filters Hydrophilic: Nipple 20 Pack 6730-0350-800 200 Pack 6730-0351-800
Hydrophobic: Nipple 3 Pack 6700-0570-800 10 Pack 6700-0571-800 50 Pack 6700-0572-800
Threaded 6700-0580-800 6700-0581-800 6700-0582-800
Note: For proper installation of adapters/probe and fittings, see page 12.
Attaching the Overflow Safety Trap (OST) CAUTION
5
To help prevent aspirate from entering the regulator, wall outlet and pipeline equipment, a safety trap should be attached prior to its use. Aspirate in the regulator, wall outlet and pipeline equipment may impair its operation. The use of the safety trap and suction filter will help prevent this and extend the life of suction equipment. 8700-0000-000 Rev.9 (03/2013)
5
Operation Locking Gland Fitting 2
Figure 2
1. Raise the sleeve and insert the safety trap into the regulator fitting. 2. Turn the trap clockwise about one and a half turns to engage the threads. The trap does not need to be screwed tight; an O-ring in the regulator fitting provides a vacuum seal. The trap should rotate freely to allow the desired nipple positioning. 3. Lower sleeve to lock trap in position. Regulator Sleeve Safety Trap
DISS fitting 3
Figure 3
1. Insert the safety trap into the regulator fitting. Situate the nipple in the desired position. 2. Turn the DISS wing nut clockwise to engage threads and tighten (there is no O-ring, so the vacuum seal depends on a tight connection). Regulator Wing nut
Safety Trap
Mode Selection 4
Figure 4 |O|O - suction is intermittent (cycled “ON” and “OFF”) and the suction level can be adjusted with the suction control knob when cycled "ON".
O - No suction is supplied to the patient.
I - Suction is continuous and can be adjusted with the suction control knob.
6
8700-0000-000 Rev.9 (03/2013)
6
Operation Setting the suction level 5
Figure 5
1. Move the mode selector switch to | (CONT).
6
Figure 6
2. Push and rotate the suction control knob until the vacuum gauge indicates the required setting.
CAUTION
The suction control knob must be completely pushed in to adjust the vacuum level. Failure to do so may damage the vacuum regulator.
Pre-Use Checkout Procedure WARNING
The Pre-Use Checkout Procedure must be performed before using the equipment on each patient. If the regulator fails any part of the Pre-Use Checkout Procedure, it must be removed from service and repaired by qualified service personnel.
Important:
All tests must be performed with supply vacuum of 66.7 kPa (500 mmHg) minimum.
Note: Pediatric/Neonatal ISU Only: Rotate suction control knob fully clockwise (increase) to verify that the suction level does not exceed 135 mmHg ± 5 mmHg (18.0 kPa ± 0.7 kPa) on the pediatric model or 100 mmHg ± 5 mmHg (13.3 kPa ± 0.7 kPa) on the neonatal model. When maximum vacuum is reached, the safety relief valve will emit an audible pulse. 1. Move the mode selector switch to O (OFF). 2. Push and rotate the suction control knob one full turn clockwise (increase). 3. Release the suction control knob. The gauge needle should not move (reading should remain at zero on units with a digital gauge). 7
8700-0000-000 Rev.9 (03/2013)
7
Operation 4. Move the mode selector switch to | (CONT). The gauge should indicate vacuum. 5. Push and fully rotate the suction control knob counter-clockwise (decrease) until it stops. 6. Release the suction control knob. The gauge needle should move to zero and remain there. (Readout should be zero on the digital gauge.) 7. Push the suction control knob and increase the suction to 90 mmHg (12.0 kPa). 8. Slowly release and push the suction control knob to create various flow rates through the regulator. Check that the suction level is maintained when the knob is fully pushed in. 9. Move the mode selector switch to |O|O (INT). Check that the intermittent timing cycles are 15 seconds ON and 8 seconds OFF both with a tolerance of ± 3 seconds by observing the gauge. Note: The PTS-ISU starts in the ON cycle. 10. Push the suction control knob and reduce the suction level to zero. 11. Set the mode selector switch to O (OFF).
Patient Setup 1. Make sure the Pre-Use Checkout Procedure has been performed.
7
Figure 7
2. Move the mode selector switch to | (CONT)
®
3. Set the prescribed suction level by pushing and turning the suction control knob.
CAUTION
The suction control knob must be completely pushed in to adjust the vacuum level. Failure to do so may damage the vacuum regulator.
4. Move the mode selector switch to O (OFF). 5. Attach tubing from the vacuum regulator to the vacuum port of the collection container. 8
8700-0000-000 Rev.9 (03/2013)
8
Cleaning and Disinfection Routine cleaning of the regulator is recommended as a standard procedure after each use. Wipe all exterior surfaces with a solution of water and mild detergent and/or an approved cleaning solution. Should misuse occur resulting in accidental flooding of the regulator, the regulator may be sterilized after cleaning using ethylene oxide (ETO). See Section 5.2 (Sterilization) of the PTS-ISU service manual. After sterilization follow the service checkout procedures in Section 8.0 (Service Checkout Procedure) of the PTS-ISU service manual. WARNINGS
CAUTION
To reduce service personnel exposure to hazardous contamination, clean and disinfect all suction equipment before disassembly.
After patient use, regulators may be contaminated. Handle in accordance with your hospital’s infection control policy.
Following sterilization with ethylene oxide, parts should be quarantined in a well ventilated area to allow dissipation of residual ethylene oxide gas absorbed by the material. Aerate parts for 8 hours at 54°C (130°F). Follow your hospital sterilization procedure.
Do not steam autoclave or liquid sterilize the regulator. Severe impairment to the operation of the regulator will result. The only acceptable method of sterilization is with gas (ethylene oxide).
Troubleshooting If the regulator does not operate and you have performed the Pre-Use Checkout Procedure, remove the regulator from service and refer to PTS-ISU service manual or refer servicing to qualified service personnel.
9
8700-0000-000 Rev.9 (03/2013)
9
Timing Cycle Adjustment Timing Cycle Adjustment WARNING: If the timing valves are turned all the way clockwise, the PTS-ISU will not cycle. Factory Settings: “ON” Cycle: 15 seconds ± 3 seconds “OFF” Cycle: 8 seconds ± 3 seconds To set the timing cycles, perform the following procedure. Note: Please have a stop watch or a Vacutimer on hand to measure the length of the timing cycles. 1. Rotate the collar which is located directly behind the suction control knob counterclockwise until loose (Figure 8). 2. Unhook the faceplate by pulling it forward until it is loose and rotates downward (Figure 9).
Collar Faceplate Figure 8
Figure 9
3. Occlude the fitting/patient port by inserting a plug or clamping the tubing or connecting a Vacutimer to fitting/patient port. 4. Move the selector switch to | (CONT). 5. Set 120 mmHg (16.0 kPa) on the regulator gauge for the adult model. For the pediatric model, set the regulator gauge to 100 mmHg (13.3 kPa) and for the neonatal model, set the regulator gauge to 80 mmHg (10.7 kPa). 6. Move the mode selector switch to |O|O (INT) with the fitting/patient port still occluded. 7. Wait 20 seconds. If the regulator does not cycle "OFF" within 20 seconds, use a flathead screwdriver to rotate the "ON" timing valve stem counter-clockwise and continue rotating until the unit cycles "OFF" (Figure 10). 10
8700-0000-000 Rev.9 (03/2013)
10
Timing Cycle Adjustment Flat-Head Screwdriver
“ON” Valve “OFF” Valve
Brass Flow Control Valve
Fitting/Patient Port
Figure 10: Setting Timing Cycles and Intermittent Flow Rate
8. Wait 20 seconds. If the regulator does not cycle "ON" within 20 seconds, use a flathead screwdriver to rotate the "OFF" timing valve stem counter-clockwise and continue rotating until the unit cycles "ON". 9. Once the regulator has completed an “ON” and “OFF” cycle, fine tune each cycle to the desired time by adjusting the corresponding valve. To increase the time, turn the timing valve stem clockwise. To decrease the time, turn the timing valve stem counterclockwise. Note: Start by using half turn increments to adjust the timing. As the target time is approached, use finer adjustments, e.g., 1/8 turn, to reach the desired timing. 10. Re-attach the faceplate by rotating it upward and snapping it in place. 11. Tighten the collar (located behind the suction control knob) by rotating it clockwise to tighten.
11
8700-0000-000 Rev.9 (03/2013)
11
Adapter/Probe Installation Installation Procedure for Adapters/Probes and Fittings CAUTIONS
Do not use any Loctite® products or any products which contain Methacrylate Ester as an active ingredient to seal the threads on the adapters/probes and fittings.
All adapters/probes and fittings should be installed properly to prevent leaks and to support the equipment when mounted. Both vacuum regulator ports are 1/8-27 NPTF tapered pipe threads. Important:
Adapters, probes and fittings seal on the thread and may have threads exposed after they have been tightened properly.
Prior to installing the adapter/probe or fitting, wrap the thread with PTFE tape or apply Dow Corning® 111, Ball Vac Kote® (37951M) or equivalent The torque range for installing adapters/probes and fittings is 4.0 ft-lb (5.4 N-m) minimum to 10.0 ft-lb (13.6 N-m) maximum. Adapters/probes and fittings which are not keyed for specific orientation, should be torqued to approximately 6.0 ft-lb (8.1 N-m). Adapters/probes and fittings that are keyed to specific orientation, must be torqued initially to 4.0 ft-lbs (5.4 N-m). Additional torque is applied only until orientation is correct.
12
8700-0000-000 Rev.9 (03/2013)
12
Warranty This product is sold by Ohio Medical Corporation, a Delaware corporation (the “company”) under the express terms of the warranty set forth below. For a period of one hundred and twenty (120) months from the date the company ships this product to the customer, but in no event for a period of more than ten years from the date of original delivery by the company to an authorized dealer, this product, other than its expendable parts (e.G., Batteries for digital gauge) is warranted to be free from functional defects in materials and workmanship and to conform in all material respects to the description for the product contained in this operation manual, if this product is properly operated under conditions of normal use, regular periodic maintenance and service is performed and repairs are made in accordance with this operation manual. The warranty period for all expendable parts of the product is sixty (60) days from the date the company ships the product to the customer. The company’s sole and exclusive obligation and customer’s sole and exclusive remedy under the above warranty is limited to repair or replacement, at the company’s option, of the defective product. The foregoing warranty shall not apply if the product has been repaired or altered by anyone other than the company or an authorized dealer; or if the product has been subjected to abuse, misuse, negligence, or accident. The company reserves the right to stop manufacturing any product or change materials, designs, or specifications without notice. This warranty is extended to only the initial customer with respect to the purchase of this product directly from the company or an authorized dealer as new merchandise. Dealers are not authorized to alter or amend the warranty of any product described in this agreement. Any statements, whether written or oral, will not be honored or be made part of the agreement of sale. THIS WARRANTY IS EXPRESSLY IN LIEU OF ANY OTHER WARRANTIES, EXPRESS OR IMPLIED, INCLUDING ANY WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. THE COMPANY SHALL NOT BE LIABLE FOR INCIDENTAL, COLLATERAL, CONSEQUENTIAL, OR SPECIAL DAMAGES INCLUDING, BUT NOT LIMITED TO, LOST PROFITS, OR LOSS OF USE. THE COMPANY’S LIABILITY, IN THE AGGREGATE, SHALL NOT EXCEED THE PURCHASE PRICE OF THE PRODUCT. In order to file a warranty claim, customer is required to return Product prepaid to the Company at 1111 Lakeside Drive, Gurnee IL, 60031 USA. As determined at the sole discretion of the Company, Products which qualify under the warranty will be repaired or replaced, at the Company’s option, and returned to customer via ground delivery at the Company’s expense. All claims for warranty must first be approved by Ohio Medical Corporation Customer Service Department. For US Domestic customer returns: [email protected] or 1-800-662-5822 (Option 3). For International customer returns: intl.customerservice@ ohiomedical.com or 1-800-662-5822 (Option 3). Upon approval the customer service department will issue a Return Goods Authorization (RGA) number. An RGA must be obtained prior to commencement of any warranty claim. FORM NO. 550020 (REV.1) 2012
13
8700-0000-000 Rev.9 (03/2013)
13
Customer Service, Distribution Center Technical Support, Sales and Service Equipment Service Center Ohio Medical Corporation 1111 Lakeside Drive Gurnee, IL 60031 USA P: 866 549 6446 P: +1 847 855 0800 F: +1 847 855 6218
© 2013 Ohio Medical Corporation. This document contains information that is proprietary and confidential to Ohio Medical Corporation. Use of this information is under license from Ohio Medical Corporation. Any use other than that authorized by Ohio Medical Corporation is prohibited.
Ohio Medical, Ohio Medical Corporation, and the Ohio Medical Corporation Logo are registered trademarks and Push-To-Set is a trademark of Ohio Medical Corporation. Dow Corning is a registered trademark of Dow Corning Corporation. Vac Kote is a registered trademark of Ball Aerospace & Technology Corp. Loctite is a registered trademark of Henkel Corporation.
8700-0000-000 Rev.9 (03/2013)