Instruction Manual
18 Pages
Preview
Page 1
GB
Instruction Manual
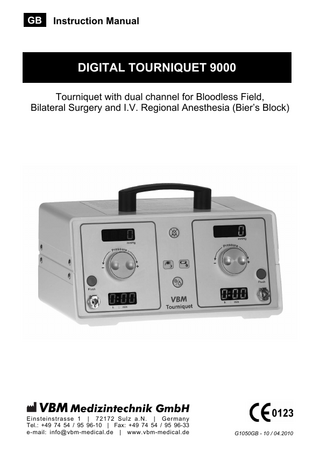
DIGITAL TOURNIQUET 9000 Tourniquet with dual channel for Bloodless Field, Bilateral Surgery and I.V. Regional Anesthesia (Bier’s Block)
VBM Medizintechnik GmbH Einsteinstrasse 1 | 72172 Sulz a.N. | Germany Tel.: +49 74 54 / 95 96-10 | Fax: +49 74 54 / 95 96-33 e-mail: info@vbm-medical.de | www.vbm-medical.de
G1050GB - 10 / 04.2010
Index Instruction Manual
Page
1
General Information
1
2
Device Delivery
1
3
Assembly and Preparations for Use: Assembly of mobile stand with basket
2
Mains Connection
2
3.1 Assembly of the battery
3
4
Technical Data
4
5
Operating Instructions Tourniquet Cuffs
4
6
Operating Instructions Tourniquet Device
5, 6
7
Safety Instructions
7
8
Operating Instructions Automatic Timer
8
9
Cleaning Instructions
8
10
EMC-table
9-12
11
Symbol definitions
12
12
Device Check
13
13
Trouble Shooting
14
14
Replacement of Parts
15
15
Spare Parts List
15
1
General Information
2
Device Delivery
Intended Use The Tourniquet 9000 is an electric unit for use with two single cuffs for Bloodless Field or Bilateral Surgery and also with double cuffs for Intravenous Regional Anesthesia (IVRA).
Device Delivery complete with: • battery in case of power failure • power cable 4 m long • colour coded extension hoses to Tourniquet Cuff • alarm system
Instruction Manual Before use, review this manual with safety instructions carefully. This device may only be used by trained medical personnel.
Availability: REF 13-12-900 Table unit
Medical Device Directive This Tourniquet Device complies with the requirements of the European Directive 93/42/EWG for medical devices.
REF 13-13-900 Table unit with universal clamp
REF 13-22-900 Table unit on mobile stand with basket
EC-certificate The design, development, production and distribution of the devices is covered by a quality system according to ISO 13485. This is confirmed by the EC Certificate issued by TÜV SÜD Product Service GmbH. For more information regarding the certificate please contact VBM or refer to the internet: http:www.vbm-medical.de Download QM-Certificates EC-Certificate Device Class IIa Note • Technical modifications reserved! • Within the EU waste management has to be carried out according to regulation 2002/96 EG (WEEE-Regulation) • In case of interference with other devices, proceed as follows: 1. Increase the distance between both devices. 2. Contact the manufacturers of the devices. • Each Tourniquet device is checked for electrical safety according to IEC 601-1 (DIN EN 60 601-1). We confirm to keep within the limits of device class I, BF-type: Protective Earth Resistance < 0.1 Ohm Earth Leakage Current N.C. < 0.5 mA Enclosure Leakage Current N.C. < 0.1 mA Patient Leakage Current N.C. < 0.1 mA • Repairs, which are not described in this Manual, have to be effected by VBM or by an authorized service.
Tourniquet Cuffs and further accessories are not included in the device delivery and have to be ordered separately. Detailed product information is available at VBM.
-1-
3
Assembly and Preparation for Use • Attach the steel hook with fixation plate (d) to the basket using two countersunk screws M5x12.
Assembly of mobile stand with basket REF 13-22-900 • Attach the lock-type rollers (3) opposite of each other to the base (1). • Attach the ordinary rollers (2) to the base (1). • Insert the support rod (4) into the base (1). • Insert cylinder hat screw M8x40 (7) with washer (6) and distortion lock (5) from below into the base (1). Fasten with hexagon socket wrench (8).
d
• Attach the basket to the square rail (f) with the steel hook (e)
e f
• Fix the square rail by means of the two hexagon socket screws to the support rod (a) • Screw the fixation plate with hexagon socket screw M6x10 to the support rod (b)
Mains Connection Plug the power cable into the back of the device and then plug the other end of the cable into the mains socket. Always use a shockproof mains socket. If local safety regulations require, connect the device to a potential equalisation mains supply or an earth connection (see the Potential Equalisation Terminal on the back panel of the device).
b
a • Screw the fixation plate with the 2 hexagon socket screws M6x10 to the lower device housing (c) To disconnect the mains supply, unplug the power plug. Should the good conditions of the set up or the position of the protection conductor be doubtful, the device has to be used only in battery mode (without mains supply).
c
-2-
3.1 Assembly of the battery As protection of the battery there is a fuse 6,3 A (T) REF 10-50-120-51 at the back panel which should be inserted prior to first use of the device – follow below instructions with photos.
3) Turn the fuse holder with a screw driver 90° clockwise until it is locked.
!! Attention !! To avoid a discharge and also a drain of the battery, the fuse should be removed in case that the device is put out of operation for more than 4 weeks. 1) Fuse and fuse holder are originally packed and fixed to the back panel via an adhesive tape.
4) Fuse in locked position.
5) After insertion of the fuse, the Tourniquet must be connected to power for 12 hours (battery will be charging). The Tourniquet can be used during charging.
2) Remove the tape and insert the fuse into the fuse holder. Put the fuse with the holder into the device.
For further information about the battery, see “Trouble Shooting / Battery” page 14. The 12 Volt NIMH battery is charged using NIMH charging technology. The charging circuit is active anytime the unit is plugged into an acceptable V~ outlet. The charger automatically sequences through several charge states based on the battery voltage and battery temperature conditions. Based on a charger test, the best charge mode is selected. No maintenance is required of the battery charging circuit. The life of the battery depends on the type of service and the storage method. Battery replacement will need to be more frequent with continued cycles of discharge/charge sequences and in case of a higher environment temperature. Infrequent short-term use of the battery and storage in a room-temperature environment will result in maximum life.
Should the good conditions of the set up or the position of the protection conductor be doubtful, the device has to be used only in battery mode (without mains supply).
-3-
4
Technical Data
5
Weight (table unit)
5.5 kg
Dimensions Height Width Depth
150 mm 320 mm 200 mm
Mains voltage
100-240 VAC
Mains frequency
50 – 60 Hz
Power consumption
75 VA
Mains fuse Battery fuse
2 x 2 A (T) 1 x 6.3 A (T)
Battery type
NiMH 12V – 3000 mAh
Protection class (IEC 601-1)
I, type BF
Operating pressure
2 bar
Regulation range
0 – 600 mmHg (5 mmHg Steps)
Regulation accuracy
+3/– 2 mmHg
Pressure accuracy
± 5 mmHg
Timer Alarm
every 30 minutes after cuff inflation (audible signal)
Pressure Alarm
audible and visual alarm indicates leak in the Tourniquet system
Noise level
< 60 dB (A)
Connection
blue / red hoses with positive locking connectors (PLC)
Data port
RS232 for optional printer for patient report
Tourniquet Cuffs
VBM offers a complete range of reusable and disposable single and double cuffs for Tourniquet 9000. VBM single and double cuffs are colour coded. Single cuffs are blue. Double cuffs are blue (proximal chamber) and red (distal chamber). Attachment and Securing of the cuff See instructions for use included with each VBM Tourniquet Cuff.
Warning Choose the adequate cuff pressure depending on cuff size and systolic blood pressure of the patient to guarantee a safe bloodless field and to avoid harm to the patient. Cuff must not be inflated for more than two hours! In case of a malfunction of the Tourniquet the cuff can be deflated by disconnecting the extension tube.
Environmental conditions: Transport/Storage
-10 ... +60°C
Operation
+10 ... +40°C 30 ... 95% atmospheric humidity without condensation
-4-
6
Operating Device
Instructions
Tourniquet
After securing the Tourniquet Cuff the Tourniquet device has to be operated as follows:
Alarm System The possible Alarm conditions will be displayed on the corresponding PRESSURE display and additionally by an audible signal.
1. Press the ON/STANDBY switch to turn the unit on. The unit will make a self-check. In case of no mains supply the yellow Battery-indicator light illuminates. Additionally the Software Version will be displayed on the PRESSURE display, afterwards 0 will be displayed on the PRESSURE display and 0:00 on the TIME display.
Possible Alarm conditions: LEAK • No Tourniquet cuff is connected, although a pressure higher than 0 mmHg is set; the alarm is activated within 8 seconds. • In case of leakage inside the Tourniquet system, which means a pressure decrease of 5 mmHg compared with the nominal value for longer than 10 seconds.
2. Connect the single cuff via the positive locking connectors to the Tourniquet. For I.V. Regional Anesthesia connect the red connecting tube to the distal (red) Cuff. 3. Inflate the single cuff by turning the pressure regulator clockwise to the desired value. During pressure adjustment the symbol * is added on the PRESSURE display. For I.V. Regional Anesthesia inflate the blue proximal chamber first. The right Timer (blue side) starts automatically. The actual cuff pressure is displayed constantly on the PRESSURE display of the device.
LOPR • In case of disconnection of the cuff the alarm is activated within 5 seconds if a pressure higher than 0 mmHg is set. HIPR • In case of high pressure inside the Tourniquet system, which means a pressure increase of 15 mmHg compared with the nominal value for longer than 5 seconds.
4. The cuff pressure in the Tourniquet Cuff can be regulated via the pressure regulator at any time. 5. During I.V. Regional Anesthesia the distal chamber of the double cuff has to be inflated (considering that analgesia already takes place). Therefore turn the distal (red side) pressure regulator to the already selected proximal pressure value. The proximal chamber can now be deflated via the pressure regulator on the right (blue) side.
If an alarm is activated, please operate according to the safety instructions. The audible signal can be switched off temporarily for 30 seconds by pressing the alarm button. Safety System
6. After the operation, the cuff has to be deflated slowly by turning the pressure regulator anticlockwise to zero. The corresponding Timer stops and displays the elapsed time.
The Tourniquet device can also be operated without mains supply. The unit switches automatically to the battery mode. This offers two advantages: • It is possible to disconnect the Tourniquet device from the mains supply in order to transport the patient with the Tourniquet in situ. • In case of a power failure the operation can be continued safely.
-5-
6
Operating Instructions Tourniquet Device
a) Color Coded hoses (blue/red) with Positive Locking Connectors easy attachment and detachment; for safe and leakfree inflation
b) Automatic Timer automatic time recording after cuff inflation; Provides elapsed inflation time for each cuff
d) Pressure Display independent pressure readings for each cuff; c) Alarm silence button Large color coded LED to switch off audible display is easy to read; alarm for 30 seconds Precise monitoring, shows actual cuff pressure
d) g)
c)
j)
h)
f) b)
e)
i)
a) e) AC Indicator light input Voltage from 100-240 VAC
f) Battery Indicator light Flushing light means charging continued light means battery mode unit operates for 4 hours with fully charged batteries
g) Precision Pressure Regulator fast cuff inflation and deflation; automatic pressure compensation, safety knob prevents accidental movement, pressure range from 0 to 600mmHg
i) ON/Standby button battery charging also in Standby mode if unit is connected to the mains. To disconnect the mains supply, unplug the power plug.
h) Flush Button (red and blue) to check for bleeding after surgery; to release drug slowly after I.V. Regional Anaesthesia
j) Built-in Data port RS232 supports optional printer for patient report; Reports cuff pressure adjustments and total elapsed cuff time on a selfadhesive label
-6-
7
Safety Instructions
Mains Connection Connect the Tourniquet device only to a grounded AC mains supply that complies with IEC requirements. Always use a three-pole cable. Connect the device to a power supply that corresponds to the input requirements indicated on the ratings plate on the back panel of the device.
Operating • Never occlude the hoses between device and cuff • Make sure that the cuff inflates properly by manual palpation. • Check the cuff pressure continuously during the operation. The PRESSURE display of the device always displays the exact cuff pressure. Any pressure deviation is indicated on the display and activates the alarm.
Should the good conditions of the set up or the position of the protection conductor be doubtful, the device has to be used only in battery mode (without mains supply).
If the alarm is activated, operate as follows: • Check the pressure constancy on the PRESSURE display. • Inspect the cuff, hoses and connections for damage. Check for firm connection. • If the alarm is still activated the device has to be inspected as described in "Service“.
Warning Do not use the Tourniquet in explosion hazarded areas, which can be caused by flammable anaesthetics and disinfectants.
Battery Recycling There are rechargeable batteries inside, which are needed for special function.
Splashing Water Protect the Tourniquet device from splashing water. The power socket on the back of the device has to be kept dry. Do not use the device if any liquid has entered the unit.
Batteries should not be disposed of into ordinary household waste. Instead, they must be recycled properly to protect the environment and also to cut down the waste of precious resources. Contaminated batteries are marked with beside symbol. The city council, the waste management authority and also local dealers inform about recycling details. Do not dive batteries into water or do not throw batteries into fire!
Attention • Make sure to select the correct cuff size. VBM offers a complete range of Tourniquet Cuffs. • Ensure that damaged cuffs and connectors are no longer used. • Make sure that the red chamber of the Double Cuff is put on distally. Before Use Check the functionality and air tightness of the Tourniquet system before each use. Put the cuff around a bottle and inflate to the maximum pressure of 600 mmHg. The alarm should not be activated within 2 minutes. Else see page 13 “Device Check”. Check the function of the alarm system. Switch on the device. Disconnect the cuff and set the pressure regulator to a value higher than 150 mmHg. The pressure alarm has to be activated within 8 seconds.
-7-
8
Operating Instructions Automatic Timer
displays time in hours
9
displays time in minutes
Cleaning Instructions
Tourniquet Device Switch off the device (ON/Standby button) and disconnect the power cable before cleaning the device! Cleaning Wipe the device with a soft and damp cloth. Disinfection Wipe the device with a cloth that has been dampened with commercially available disinfectants in low concentration. Never immerse the Tourniquet unit in liquids!
Time controlling • The timer is a fully automatic timer receiving its activation from the pressure regulator. The timer starts automatically after inflation of the cuff (pressure regulator ≥ 100 mmHg). This is indicated by the flashing colon. The timer now shows the elapsed time (minutes and hours only).
Sterilisation Do not sterilise the Tourniquet unit!
• Every 30 minutes an audible signal and flashing display reminds the operator regarding the elapsed time. The blood occlusion time must not exceed two hours.
Tourniquet Cuffs Cleaning, Disinfection, Sterilisation Follow the instructions for use included with each VBM Tourniquet Cuff.
• After cuff deflation, the timer stops automatically and the colon stops to flash. Now the total elapsed inflation time can be recorded.
-8-
10 EMC Tables Guidance and manufacturer’s declaration – electromagnetic emissions The Tourniquet 9000 is intended for use in the electromagnetic environment specified below. The customer or the user of the Tourniquet 9000 should assure that it is used in such an environment Emissions test Compliance Electromagnetic environment - guidance RF emissions Group 1 The Tourniquet 9000 uses RF energy only for its internal function. Therefore, its CISPR 11 RF emissions are very low and are not likely to cause any interference in nearby electronic equipment RF emissions The Tourniquet 9000 is suitable for use in all establishments, including domestic CISPR 11 Class B establishments and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes. Harmonic emissions IEC 61000-3-2 Voltage fluctuations/ flicker emissions
Class A
Complies
IEC 61000-3-3
-9-
10 EMC Tables Guidance and manufacturer’s declaration – electromagnetic immunity The Tourniquet 9000 is intended for use in the electromagnetic environment specified below. The customer or the user of the Tourniquet 9000 should assure that it is used in such an environment. Immunity test IEC 60601 Compliance level Electromagnetic environment test level guidance Electrostatic ±6 kV contact ±6 kV contact Floors should be wood, concrete discharge (ESD) or ceramic tile. If floors are cov±8 kV air ±8 kV air ered with synthetic material, the IEC 61000-4-2 relative humidity should be at least 30%. Electrical fast ±2 kV for power ±4 kV for power Mains power quality should be transient/burst supply lines supply lines that of a typical commercial or hospital environment. IEC 61000-4-4 Surge ±1 kV differential ±1 kV differential Mains power quality should be mode mode that of a typical commercial or IEC 61000-4-5 hospital environment. ±2 kV common ±2 kV common mode mode <5 % UT Mains power quality should be Voltage dips, short <5 % UT that of a typical commercial or interruptions and (>95 % dip in UT) (>95 % dip in UT) hospital environment. If the user voltage variations on for 0,5 cycle for 0,5 cycle of the Tourniquet 9000 requires power supply input lines 40 % UT ( 60 % dip 40 % UT ( 60 % dip continued operation during power mains interruptions, it is in UT ) for 5 cycles in UT ) for 5 cycles recommended that the TourniIEC 61000-4-11 70 % UT ( 30 % dip 70 % UT ( 30 % dip quet 9000 be powered from an in UT ) for 25 cycles in UT ) for 25 cycles uninterruptible power supply or a battery. <5 % UT ( >95 % <5 % UT ( >95 % dip in UT ) for 5 s dip in UT ) for 5 s Power frequency (50/60Hz) magnetic field
3 A/m
3 A/m
Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment.
IEC 61000-4-8
- 10 -
10 EMC Tables Guidance and manufacturer’s declaration – electromagnetic immunity The Tourniquet 9000 is intended for use in the electromagnetic environment specified below. The customer or the user of the Tourniquet 9000 should assure that it is used in such an environment. Immunity test IEC 60601 test level Compliance Electromagnetic environment - guidlevel ance Portable and mobile RF communications equipment should be used no closer to any part of the Tourniquet 9000, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter. Recommend separation distance Conducted RF
3 Vrms
IEC 61000-4-6
150 kHz to 80 MHz
Radiated RF
3 V/m
IEC 61000-4-3
80 MHz to 2,5 GHz
3Vrms
d = 1,2√P
10 V/m
d = 0,35√P 80 MHz to 800 MHz d = 0,7√P 800 MHz to 2,5 GHz where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in metres (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey, a should be less than the compliance level in each frequency range. b Interference may occur in the vicinity of equipment marked with the “Non Ionizing Radiation” symbol
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the Tourniquet 9000 is used exceeds the applicable RF compliance level above, the Tourniquet 9000 should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting relocating the Tourniquet 9000 b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
- 11 -
10 EMC Tables Recommended separation distances between portable and mobile RF communications equipment and the Tourniquet 9000 The Tourniquet 9000 is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the Tourniquet 9000 can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the Tourniquet 9000 as recommended below, according to the maximum output power of the communications equipment. Rated maximum output power of Separation distance according to frequency of transmitter transmitter m 800 MHz to 2,5 GHz 80 MHz to 800 150 kHz to 80 MHz W MHz d = 2,3√P d = 1,2√P d = 1,2√P 0,01 0,12 0,12 0,23 0,1 0,38 0,38 0,73 1 1,2 1,2 2,3 10 3,8 3,8 7,3 100 12 12 23 For transmitters rated at a maximum output power not listed above, the recommended separation distance d in metres (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
11 Symbol definitions Symbols and abbreviation
Definitions
Symbols and abbreviation
Definitions Unit ‚ON’ (only for a part of the unit)
Refer to Instruction manual
Standby Mode Equipotential Temporary acoustic signal switch off Attention
Battery condition indicator Signal output Power supply operation indicator
Electrical device, which is delivered after 13.08.2005 in the EU.
Type BF Equipment
Electrical Hazard Sign, that a material is a part of a recycling process. It is not allowed to dispose batteries in the domestic refuse.
Grounding symbol, Protection class I, according IEC 60417-5019 - 12 -
12 Device Check Function and Leak test
Repairs
Execute following test if necessary (see "Safety Instructions - Before use" or "Troubleshooting"):
Repairs which are not described in this Manual may only be carried out by QUALIFIED SERVICE PERSONNEL. Otherwise VBM cannot be held responsible for safety, reliability and performance of the device. VBM does not accept any warranty claims if the user or an unauthorised service agency has attempted to effect repairs which are not described in this Service Manual. To ease repair of the device return it together with a detailed description of the defect. As a protective measure for the safety of VBM staff return the device or cuffs completely cleaned and disinfected (see “Cleaning, Disinfection and Sterilisation”). The VBM Service is entitled to refuse repairs of contaminated items for safety reasons.
1. Connect the device to the mains supply. The AC Indicator light illuminates green. 2. Put the cuff around a bottle. 3. Connect the red extension hose to the cuff. 4. Switch on the device. 5. Now it must be possible to adjust any desired pressure value (5 mmHg steps) with the pressure regulator. The pressure value has to be displayed. 6. Set the pressure to 600 mmHg. The corresponding Timer starts automatically. 7. Press the red flush button and the pressure decreases immediately. Release the red flush button and the pressure goes back to 600 mmHg. 8. The alarm should not be activated within 2 minutes. In case of a leak alarm follow the instructions on page 14. 9. Set the pressure to 0 mmHg. The corresponding Timer stops automatically and shows the elapsed time. 10. To check the right (blue) side, repeat the steps 29 analogous.
- 13 -
13 Trouble shooting Failure/Defect
Cause/Removal
Mains Switch • AC Indicator light does not illuminate • “defl cuff” is shown in the pressure display
LEAK-Alarm • Leakage (LEAK/LOPR) in the system (device with cuff).
¾ Fuses on the rear panel are defective. Check the fuses and replace them if necessary (page 15). ¾ It is not possible to switch off the device while the cuff is inflated. In order to switch off the device the cuff needs to be deflated.
¾ No cuff is connected, although a pressure higher than 0 mmHg is set (both sides). Turn the pressure regulator to 0 mmHg. ¾ Cuff is damaged. Check the device with another cuff (page 13 “Function and Leakage test”). Replace the cuff if necessary. ¾ See “Leakage inside the device”.
• Leakage inside the device
¾ Washer of male locking connector is porous or missing. Replace the washer.
Battery • BATT LOW • BATT FAIL
¾ Battery voltage is too low to ensure an operation time of 2h 30 min. Press alarm button to confirm that the procedure can continue. (charge battery) ¾ Fuse at the back panel is defect or missing. Please see instructions at page 3. ¾ Battery defective. Charge battery for at least 12 h. In case that the signal still appears, an QUALIFIED SERVICE PERSONNEL has to replace the battery.
Pump • Pump does not start
¾ Set the device in motion again. If the failure persists, the device needs to be returned.
- 14 -
14 Replacement of Parts Replace the fuses
a 1. Pull off the mains plug. 2. Push up the shackle at the power socket on the rear panel with a screw driver (a). 3. Fuse socket is loose and can be removed. 4. Replace only with the same type and rating of fuse. Mains fuse 2 A(T) 20x5 mm REF 20-15-772
15 Spare Parts List Coil Extension Hose, max. stretch length 3.0 m, with positive locking connectors – colour: red – colour: blue
REF 20-20-742 REF 20-20-744
REF SLZM30 Positive Locking Connector, male
REF SLZF40 PositiveaLocking Connector, female, self-locking
- 15 -