VIASYS Healthcare
Infant Flow LP System Instructions for Use Ver E
Instructions for Use
15 Pages
Preview
Page 1
Infant Flow™ LP System Instructions for Use
Vyaire Medical, Inc. 26125 North Riverwoods Blvd. Mettawa, IL 60045 USA 1-833-327-3284 [email protected] www.vyaire.com
EC
REP
CE Mark
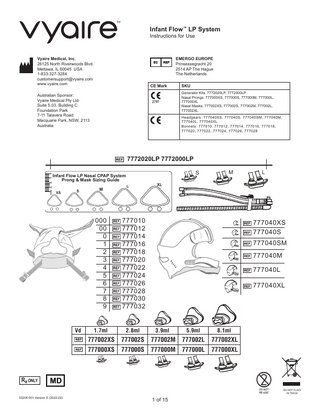
EMERGO EUROPE Prinsessegracht 20 2514 AP The Hague The Netherlands SKU Generator Kits: 7772020LP, 7772000LP Nasal Prongs: 777000XS, 777000S, 777000M, 777000L, 777000XL Nasal Masks: 777002XS, 777002S, 777002M, 777002L, 777002XL
Australian Sponsor: Vyaire Medical Pty Ltd Suite 5.03, Building C Foundation Park 7-11 Talavera Road Macquarie Park, NSW, 2113 Australia
Headgears: 777040XS, 777040S, 777040SM, 777040M, 777040L, 777040XL Bonnets: 777010, 777012, 777014, 777016, 777018, 777020, 777022, 777024, 777026, 777028
7772020LP 7772000LP 0
S
5 10
Infant Flow LP Nasal CPAP System Prong & Mask Sizing Guide
15 20mm
XS
S
Vd
M
L
M
L
XL
000 00 0 1 2 3 4 5 6 7 8 9
777010 777012 777014 777016 777018 777020 777022 777024 777026 777028 777030 777032
1.7ml
2.8ml
3.9ml
5.9ml
8.1ml
777002XS
777002S
777002M
777002L
777002XL
777000XS
777000S
777000M
777000L
777000XL
777040XS 777040S 777040SM 777040M 777040L 777040XL
Rx ONLY DO NOT PLACE IN TRASH
33204-001 Version E (2022-02)
1 of 15
2
26 25
8 27 2 29 30 31
32
2
3
1
2
3
3
2
23
24
1
20 21®
22
HEADGEAR
Infant Flow™ LP System
2
1
XDCR
mm
mm
Infant Flow SiPAP R
NCPAP/ Pres Low L/min
3
Pres High L/min
%O 2 40
30
21
XDCR
50
60 70 80
90 100
PPROX
15.0 13.0 11.0 9.0 (cmH20)
7.0 5.0 3.0 1.0
BONNET
4.0
33204-001 Version E (2022-02)
2 of 15
6.0
8.0
10.0
(LPM)
12.0
14.0
16.0
18.0
2
3
Intended Use:
For use with the infant flow siPAP, infant flow, Airlife drivers, bellavista ventilator (with neo option) and the ix5 series ventilators. This device, consisting of a generator, prongs, masks, bonnet and headgear, is intended to provide CPAP to spontaneously breathing infants showing signs of respiratory distress.
Warning:
• Use this product only as directed in the product literature to reduce the risk of nasal 2 irritation, septal distortion, skin irritation, and pressure necrosis. 3 • To be used by a trained practitioner, under the direct supervision of a qualified physician. • Only use the Infant Flow LP generator with variable flow nCPAP drivers. • Do not over tighten the fixation straps.
Notes:
• Select the appropriate size nasal mask to minimize leaks and dead space. • Select the appropriate size nasal prongs; if between sizes, select the larger size. • Application of an incorrectly sized prong, mask, bonnet, or headgear will affect stability of the generator. Consider alternating the use of prong and mask interfaces at set intervals to change pressure points on the infant’s face. • Continuously monitor patient’s respiratory status, (respiratory rate, heart rate, oxygen saturation). • Cover both ears evenly; ensure the ears are not folded. • Adjust the straps to stabilize the generator and maintain a seal at the nose using the least tension possible. • Recommend humidification be used with nCPAP systems. • The Infant Flow LP has a built in “pop-off,” which is activated if the drive pressure exceeds 60 cmH2O. • For expiration dates, reference product labeling.
Duration of Use:
• Generator Assembly shall operate as specified for a continuous use of up to 30 days. • Patient Interface (Prongs and Masks) shall operate as specified for a continuous use up to 14 days. • Fixation Devices (Headgear and Bonnets) shall operate as specified for a continuous use of up to 2 days or 24 on/off cycles (based on normal patient inspection as frequently as every 2 hours over the 48 hour period).
Final Check and Routine Inspection Inspect the system after set-up and routinely at least every 3 to 4 hours to …
• Ensure the patient is receiving the prescribed level of CPAP. • Ensure the generator is stable, secure, and not pulling upward on the nose. • Check for deformation or irritation to the nose or surrounding tissue. • Ensure that the patient’s septum is clearly visible when using prongs. • Ensure that the patient’s eyes are clearly visible and that the nares are not blocked, when using masks. • Inspect the fixation device and straps for proper tension and adjust as needed to maintain a proper fit. • Monitor the patient for gastric insufflation and abdominal distension. • Monitor for excessive condensation in circuit and generator.
For single use only. Re-use may degrade the performance of the product or contribute to cross contamination. Disposal Instruction: Dispose in container that is clearly labeled, leak-proof, and color coded per local standards and state environmental regulations. Protected by Patents: US7578294, ZA200804775 US7762258, ZA200804774 US7640934, ZA200804773
Any serious incident that has occurred in relation to the device should be reported to the manufacturer at [email protected] and the competent authority of the Member State in which the user and/or patient is established.
33204-001 Version E (2022-02)
3 of 15
Customer and Clinical Support Product, Accessories, and Parts Ordering 1-833-327-3284 [email protected] General warning, as defined in ISO7010-W001 Caution Manufacturer Indicates the medical device manufacturer, as defined in EU Directives 90/385/EEC, 93/42/EEC, and 98/79/EC Date of manufacture Size EC
REP
Authorized representative in the European Community. Indicates the authorized representative in the European Community. Device meets European directive 93/42/EEC concerning medical devices. Device Meets Regulation (EU) 2017/745 concerning medical devices. Catalogue Number Labels the equipment part number. WEEE Labels the device as NOT disposable through the normal waste stream (incineration, for example)
DO NOT PLACE IN TRASH
Rx ONLY
Caution: Federal law restricts this device to sale by or on the order of a physician. Do not re-use. Medical Device Exp. Date
Not made with natural Not made with natural rubber latex. rubber latex
Consult instructions for use. Not made with PHT-DEHP.
33204-001 Version E (2022-02)
14 of 15