48 Pages
Preview
Page 1
Page 2/48
PMAM PMRM HANDPIECES (EN) 1. TYPE Straight and angled handpieces with external spray, without light for microsurgery. 2. SYMBOLS USED CE Marking with the number of the notified body. Rx Only
Caution: Federal law (USA) restricts this device to sale by or on the order of a licensed healthcare practitioner. CAUTION! Refer to documents
the
Consult the documents
accompanying
accompanying
Materials to be recycled The disposal and/or recycling of materials must be performed in accordance with the directives and the legislation in force. Sterilizable in autoclave up to the specified temperature Cleaning in machine authorized Manufacturer Movement indicated
in
the
direction
Movement fully to the stop, in the direction indicated
3. INTENDED USE Medical device for professional use, as part of surgical operations in the areas of othorhynolaryngology, otoneurology, plastic surgery, reconstruction, head and neck surgery. 4. CONTRAINDICATION None known as of today 5. PRECAUTIONS – WARNINGS 5.1.Warnings, Precautions for use For additional information, please contact Bien-Air Surgery SA at the address indicated on the backcover of this document. CAUTION: Use adequate irrigation and avoid excessive pressure on the tool. The use of a tool without irrigation and with excessive pressure may cause an inordinate amount of heat buildup resulting in a thermal injury to tissue. See tools instruction for use for further information. The device and its accessories should be used only by duly trained and competent medical personnel, in particular in compliance with the legal provisions in force regarding occupational safety, health and accident prevention measures, and the present user manual. According to these measures, the user has the following obligations: - To only use devices in perfect working condition. In the event of irregular operation, excessive vibrations, abnormal overheating or other signs suggesting malfunctioning of the device, work must be suspended immediately. In this case, contact a repair centre approved by Bien-Air Surgery. - Make sure that the device is used only for the purpose for which it is intended, protect yourself, patients and third parties from all danger and avoid contamination by the product.
with the intended use is unauthorized and may prove dangerous. This medical device complies with the European legal provisions in force. Install the handpiece on an appropriate mounting to prevent risks of injury or infection for you, the patient or third parties. Use only Bien-Air Surgery SA original maintenance products, accessories and/or spare parts. The use of other products, accessories or parts could void the guarantee and/or endanger the patient or the operator. 5.2.Environmental protection and indications for device disposal This equipment must be recycled. The disposal and/or recycling of materials must be performed in accordance with the directives and the legislation in force. The user can return the device to his distributor or call directly on a firm accredited for the treatment and recovery of this type of equipment (European directive 2012/19/EU). 6. TECHNICAL DATA Environmental conditions: Work
Temperature: +10°C to +30°C (+50°F to +86°F) Relative humidity:
20% to 80%, including condensation
Atmospheric 700 hPa to 1060 hPa pressure: Transport Temperature: -25°C to +70°C (-13°F to +158°F) Relative humidity:
10% to 100%, including condensation
Atmospheric pressure:
500 hPa to 1060 hPa
The device and its accessories are designed solely for medical treatment. Any use not in conformance Page 3/48
Storage
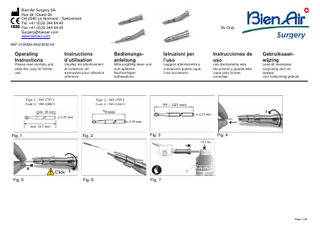
7.3.Type of bur (Fig. 1-3) Diameter of shaft 2.35 mm (0,09 in) type 2, in accordance with ISO 1797-1 - Total length 44.5 mm (1.75 in) (code 4, in accordance with ISO 6360/1) - Total length 70 mm (2.75 in) (code 6, in accordance with ISO 6360/1) - Special lengths 95 mm and 125 mm.
Temperature: +10°C to +30°C (+50°F to +86°F) Relative humidity:
20% to 80%, including condensation
Atmospheric pressure:
500 hPa to 1060 hPa
Transmission ratios: 1:1 blue ring 1:2 red ring
CAUTION: Do not exceed the stipulated diameters and speeds. Bien-Air Surgery SA declines all responsibility if burs of another brand are used.
Noise level: <70 dBA (according to IEC 60601-1) Size: Ø 20 mm Period of operation: See Chap 7.7 Classification: IIa as per 93/42 EEC directive Sterilization: See Chap 8.5 For other technical information, see below table: For further information concerning microsurgery instruments and their accessories, please contact your Bien-Air Surgery SA local distributor or consult our homepage www.bienair.com. 7. USE 7.1.Type of coupling The most commonly used coupling in the world as per ISO Standard 3964. 7.2.Drive By any motor with ISO 3964 type coupling. Handpiece reference
REF 1600206 REF 1600207 REF 1600295 REF 1600336 REF 1600337 REF 1600612
Designation
PMRM 1122-70 PMAM 1122-70 PMAM 1221-70 PMAM 1122-95 PMAM 1122-125 PMRM 1124
Max. bur length (mm) 70 70 70 95 125 44.5
Ratio
1:1 1:1 1:2 1:1 1:1 1:1
Maximum speed for all Bien-Air Surgery SA burs: Diameter Speed < Ø 4,5 mm 80,000 rpm Ø 5,0 mm 60,000 rpm Ø 5,5 mm 50,000 rpm Ø 6,0 mm 40,000 rpm Ø 6,5 mm 30,000 rpm Ø 7,0 mm 20,000 rpm 7.4.Insertion and removal of a bur Turn the ring completely in the direction of arrow and insert the bur up to the thrust stop: A: unlocking (fig. 4) B: insert the bur up to the thrust stop C: locking up to the thrust stop (until you hear a “clic”) (fig. 5). Max. Motor speed (rpm) 80’000 80’000 40’000 80’000 80’000 40’000
Max. bur speed (rpm) 80’000 80’000 80’000 80’000 80’000 40’000
Length – handpiece without bur (mm) 111 128 131 152 182 85
7.5.Check Pull on the bur and check that it is correctly in position. CAUTION: The bur must be in perfect condition: shape, surface condition and cut. The user is fully responsible for: - Ensuring that the bur meets the Bien-Air Surgery SA specifications. - Ensuring that it rotates without vibration at the speed at which it will be used. CAUTION: Comply with maximum lengths by always inserting the bur as far as possible into the locking mechanism. If a bur is operated at high speeds when incorrectly mounted (i.e. not fully inserted into the locking mechanism, or being longer than the values specified above) this can cause a centrifugal force which may bend or break the bur. CAUTION: Do not exceed the maximum operating speed authorised by the bur manufacturer. 7.6.Precautions – warnings CAUTION: Before using the instrument, place it in operation at moderate speed with a tool in the bur locking mechanism for a few second, so as to spread the lubricant and remove any excess.
Handpiece weight
The device must not be started without a bur inserted into the chuck.
(g)
Never mount an instrument on a rotating motor.
84 94 100 97 104 69
Never adjust the clamping/unclamping ring while the instrument is rotating, since this could damage the mechanism or destroy the motor. Never close the chuck mechanism without the bur in place, or it may get damaged. Page 4/48
Comply with the recommendations for use, in accordance with the instructions of the tool manufacturer. An inappropriate maintenance, use of inappropriate burs, or an instrument not properly closed, can cause a fast heating of the handpiece and create a risk of severe burn. 7.7.Period of operation To avoid overheating leading to burns (temperatures between 41°C (106°F) and 48°C (118°F) on the outer surface of the handpiece), the following rules should be complied with: a) Limit the maximum speed of rotation according to the bur diameter as indicated on the packaging and/or in the present operating instructions. b) Do not exceed the maximum speed of rotation permissible for the handpiece. c) Adequate irrigation is strongly recommended.
Before use, please comply with the present section: - Clean, lubricate and sterilize the handpiece before first use. - Clean, lubricate and sterilize the handpiece before each further use. - After each use, clean and sterilize the handpiece as quickly as possible. Precautions of use Hospital procedures must be followed. The universal precautions must be complied with by hospital personnel working with contaminated or potentially contaminated medical instruments. Pointed and sharp instruments should be handled with great caution.
8. CLEANING / MAINTENANCE / STERILIZATION
Agents required for cleaning: • Detergents Manual cleaning of the handpieces has been validated using an enzymatic pH neutral detergent (ASP Enzol®). Automated cleaning has been validated using an alkaline detergent (Neodisher MediClean Forte®, pH ca 10.5) and an enzymatic detergent (Steris Prolystica® 2X Concentrate Enzymatic Presoak and Cleaner).
CAUTION: Do not place the handpiece in an ultrasonic bath.
Detergents should be used at the concentration, temperature and for the duration recommended by the detergent’s manufacturer.
CAUTION: Never submerge the handpiece in physiological salt water solutions (NaCl solution), because prolonged contact may cause corrosion.
To remove physiological liquid inside the instrument or the spray tube, use “Aquacare” from Bien-Air Surgery SA.
During the surgical operation, if the irrigation tube becomes clogged, use the cleaning wire and a long cleaning curette to clear.
CAUTION: Chemical disinfection of the handpieces is not recommended due to possible negative effects on the lifetime of the devices and possible residues of disinfectants. CAUTION: These devices are delivered “non sterile”.
CAUTION: Do not use detergents that are corrosive or contain chlorine, acetone or bleach, aldehydic products or alcohols. • Lubricant: Exclusively use “Lubrifluid” from Bien-Air Surgery SA.
• Brush / cleaning gun The brushes should be non-aggressive to avoid damaging the device. Preferably use nylon brushes with flexible or soft bristles. Use the cleaning gun with the appropriate nozzles for the various ducts. If you have any additional questions regarding reprocessing procedures, instructions for reusable devices etc., please contact Bien-Air Surgery SA. 8.1.Point of use cleaning CAUTION: Initial cleaning should be performed at the point of use and as soon as possible after the completion of the surgical procedure. CAUTION: Point of use cleaning must be followed by manual or automatic cleaning. This operation is important in order to facilitate subsequent cleaning stages (it prevents dirt from drying and sticking to the equipment). As soon as you have finished using the handpiece, proceed as follows: - Separate all components: remove rotary instrument from the handpiece, separate external irrigation tube from the handpiece. - Separate the handpiece from the micromotor. - Leave locking ring in open position. - Rub the outside surface of the handpiece and of the irrigation tube with non-woven towelettes (pre-soaked in water). - Spray the inside of the irrigation tube in the direction it is used with “Aquacare” or inject tap water with a syringe. Check that water can flow through the duct. If obstructed, unblock the canal. - Ensure that the handpieces do not dry before manual or automatic cleaning by wrapping them in non-woven towelettes (pre-soaked in water) - Manual or automatic cleaning must be done max 4h after the point of use. Page 5/48
8.2.Manual cleaning For external irrigation tubes: - Rinse the irrigation tube under running tap water (cold, max 20°C / 68°F) and brush with nylon soft bristles for at least 15 seconds. Spray the inside of the tube for 1 second with "Aquacare" or inject tap water with a syringe. - Soak the irrigation tube in lukewarm ultrasonic bath with enzymatic pH neutral detergent (like Enzol®) for at least 3 minutes. - Thoroughly rinse under running tap water (use critical water for neurosurgical application refer to AAMI TIR34:2014 “Water for the reprocessing of medical devices.) for 10 seconds. Use a syringe with water or “Aquacare” to remove detergent solution from inside the tube. - Blow dry the irrigation duct with pressurized air For handpieces: - Rinse the handpiece under running tap water (cold, max 20°C / 68°F) and brush with nylon soft bristles for at least 30 seconds. - Soak the handpiece in a lukewarm solution for at least 5 minutes with an enzymatic pH neutral detergent (like Enzol®). - Shake it inside the detergent and open and close the locking ring at least 3 times to remove air from the device and ensure the detergent solution reaching all lumens - Thoroughly brush the outside with nylon soft bristles for at least 30 seconds. Continue brushing until soil is no longer visible. - Using a 3mm diameter nylon brush, thoroughly brush back and forth inside the handpiece with at least 3 full movements. - Remove the handpiece from the bath and thoroughly rinse vertically under running tap water (use critical water for neurosurgical application refer to AAMI TIR34:2014 “Water for the reprocessing of medical devices.) from both ends for at least 30 seconds, opening and closing the locking ring at least 3 times. At the end leave it in open position.
Drying: - Dry it by wiping it with a clean and dry non-woven towelette. - If the handpiece is not immediately sterilized, perform dynamic drying under ventilation, at about ~90°C (194°F), for a minimum of 25 minutes. Or 8.3.Automatic cleaning Pre-cleaning: - Rinse the handpiece under running tap water (15-25°C / 59-77°F) and brush the outside and all accessible lumens with nylon soft bristles (diameter of 3mm for lumens) for at least 30 seconds until soil is no longer visible. - During brushing, open and close the locking ring at least 3 times to ensure reaching all accessible areas. At the end, leave the locking ring in open position. - Rinse the irrigation tubes under running tap water (15-25°C / 59-77°F) and brush the outside with nylon soft bristles for at least 30 seconds. Continue brushing until soil is no longer visible. - Spray (1 sec.) the inside of the irrigation tubes with “Aquacare” or inject tap water with a syringe. - Pre-cleaning has to be directly followed by final automated cleaning. Place the disassembled handpiece and irrigation tubes in the appropriate washer/disinfector basket and treat via a standard instrument washer/disinfector cycle. Use exclusively a validated washer/disinfector (ISO 15883). Stages of the automatic washer/disinfector cycle: Pre-wash: Cold tap water (<45°C / 113°F) for minimum 2 minutes
Washing: Hot tap water (50- 60°C /122- 140°F) with alkaline detergent (Neodisher MediClean Forte®) or enzymatic detergent (Steris Prolystica® 2X Concentrate Enzymatic Presoak and Cleaner) for minimum 5 minutes Neutralization: Cold tap water (<45°C / 113°F) for minimum 2 minutes Rinsing: Cold critical water acc to AAMI TIR34 (<45°C / 113°F) for minimum 2 minutes. Thermal disinfection by rinsing: Hot critical water acc to AAMI TIR34 (90°C / 194°F) for minimum 5 minutes. Operator is Responsible for the implemented value A0 according A0 concept describe in EN ISO 15883 (For example A0 600 90°C(194°F)/1min.) Ventilated dynamic drying: 70°C (158°F), for minimum 22 minutes. Comments: - Comply with the washer/disinfector loading instructions provided by the manufacturer. - Make sure that all the instruments have been correctly attached to the baskets. - Make sure that the instruments do not touch one another and that internal canal are properly rinsed. - Remove the instruments from the washer or disinfector immediately after the machine stops and move on quickly to lubrication and sterilization, to avoid corrosion. 8.4.Inspection, lubrication and testing Carefully inspect each part to make sure that all visible contamination has been eliminated. Check in particular that the ducts are clear. Where there is contamination, repeat the cleaning process. Where there is moisture, use the air gun to dry it.
Page 6/48
After each cleaning operation and before each sterilization, lubricate the instrument with “Lubrifluid” from Bien-Air Surgery SA. To absorb any excess lubricant, apply a cloth over the instrument's apertures. Insert the “Lubrifluid” spray plastic end fitting in the rear of the instrument's handle (Fig. 7) and actuate the spray for about 0.5 seconds. Check the devices for any visible signs of deterioration or damage. Check the presence and integrity of all visible seals. Check the action of moving parts: Insert a clean rotary instrument onto the handpiece and close the locking ring (Fig. 5). Check that the rotary instrument remains in place when pulled. Holding the rotary instrument between your thumb and index finger, spin the handpiece. The handpiece shall spin freely (more than 3 turns). If it does not, there is danger of burning the patient and you should send the handpiece to your retailer or to Bien-Air Surgery SA to be repaired. Remove the rotary instrument and leave the locking ring in the open position (Fig. 4). Packing for sterilization: CAUTION: Do not reassemble the handpiece with any external irrigation tubes prior to sterilization. Separate packing: Immediately insert the handpiece in individual wrapping, such as a paper/plastic pouch or sterilization wrap for steam sterilization. Or Pack in stiff boxes and trays with defined, preconfigured lids and apertures and wrap the stiff boxes or tray. In the USA, FDA approved sterilization wraps, pouches or containers must be used.
8.5.Sterilization Sterilization by vacuum steam is recommended. The following sterilization parameters, using a PreVac cycle, have been validated by the legal manufacturer to provide a sterility assurance level (SAL) of 10-6.
into direct contact with patients suspected of or confirmed as having TSE/CJD.
Only legally marketed, FDA cleared sterilizers, sterilization wrap/pouches, biological indicators, etc. should be used by the end-user for packaging terminally sterilized devices. Water quality according to AAMI ST79.
CAUTION: Do not exceed a temperature of 138°C (280°F).
Temperature
132°C (269°F)
134°C2 134°C2 (273°F) (273°F)
Time
4 Min.
3 Min.
Drying
30 Min.3
1
2 3
135°C (275°F)
18 Min.1 3 Min.
Parameters recommended by the World Health Organization for treating instruments in the event of contamination by Non-Conventional Transmissible Agents (NCTA). Parameters recommended by Bien-Air Surgery SA. Not for users in US healthcare facilities Refer to sterilizer manufacturer recommendations for drying times per load configuration.
After sterilization, let the device cool down to room temperature without forced cooling. For USA only: Use sterilization cycles consistent with the cycle specifications in ANSI/AAMI ST79 “Comprehensive guide to steam sterilization and sterility assurance in health care facilities”. Since no reprocessing methods have been validated for removing transmissible spongiform encephalopathy (TSE) agents from medical devices, this device should not be used for patients with known or suspected TSE agent disease, including CJD and vCJD. The legal manufacturer recommends incinerating devices that have come
The sterilizer manufacturer instructions concern-ing operation and load configuration should be complied with explicitly.
CAUTION: Never rinse instruments in cold water to cool them. 8.6.Storage Bien-Air Surgery SA strongly advises storing only sterilized devices so as to reduce the risks of corrosion. Ambient conditions of storage after sterilization - Store the equipment in a clean, dry place at ambient temperature (10–30°C (50–86°F), 20– 80% humidity). - Do not expose the equipment to direct sunlight. - Do not expose the equipment to permanent X-ray irradiation. - Do not store the equipment in places that could be subject to liquid splashes. - Do not store the equipment in the following ambient conditions: - Dust - Saline or sulphurous atmosphere - Do not store the equipment in a location where there is a risk of release of flammable gases. Shelf life of sterilized instruments The shelf life of stored sterilized instruments depends on the type of packaging used and the storage conditions (refer to the DIN 58953 standard, section 9, or the existing local regulations). 9. MAINTENANCE No component of the handpiece may be changed by the user. Never disassemble the handpiece.
Page 7/48
For all servicing and repairs, we recommend that you contact your dealer or Bien-Air Surgery SA directly. Bien-Air Surgery SA invites users to have their dynamic instruments checked or serviced at least once a year. Hygiene For the safety of the repair center’s personnel, the instrument should be cleaned and sterilized completely before being returned for repair. If that proves impossible, for example because a disinfection or sterilization would make the instrument completely unusable, clean the instrument as carefully as possible and mark it accordingly to indicate that it has not been decontaminated. 10. MALFUNCTIONS AND ERRORS Use the table below to solve any problem encountered. If the problem is insoluble, stop using the product and contact a repair center approved by Bien-Air Surgery SA. Problem There is no transmission of movement.
Solution Check that the motor by itself operates. Check that there is a bur in the locking mechanism and that it is in “clamp” position (Fig. 5).
Impossible to insert the bur in the instrument
Check that the chuck mechanism is in open position (Fig. 4). Check that there is no part jammed inside the clamp and that the latter is clean. Check that the stem of the bur is in sound condition and of the correct diameter.
The bur does not rotate freely
Check that the locking mechanism ring is fully inserted (Fig. 5).
Check that the part is clean and lubricated with "Lubrifluid". The instrument heats abnormally.
Check that the part has been correctly cleaned and lubricated. Check that the handpiece is fully closed.
Excessive vibration
Stop the motor and pull on the bur to check that it is correctly in position, or change/reduce the speed of rotation.
11. GENERAL TERMS OF GUARANTEE 11.1.Generalities Bien-Air Surgery SA endeavors to provide its customers with products and devices of impeccable quality, which it guarantees within the limits of the present general terms and the particular agreements signed, against any operating fault, material or manufacturing defect. The guarantee period is 12 months from the date of invoice. In general, the guarantee does not exempt the customer from the obligation of obtaining information from Bien-Air Surgery SA in case of doubt and in particular when the product is used in conditions not explicitly provided for originally. The buyer is obliged to check the goods received within 8 days following their receipt. If the goods are not checked within the period mentioned above, the customer shall be deemed to have accepted the goods, barring hidden defects. The defect notice must be received in writing by Bien-Air Surgery SA within the aforementioned period and must contain the customer's name, the date of purchase, and the product reference and serial number. In the event of claims, Bien-Air Surgery SA or its authorized representative shall perform product
repair or replacement free of charge, after analyzing the justification for the claim. All other claims of whatsoever kind, and in particular claims for damages, are excluded. Bien-Air Surgery SA shall not be held responsible for damage or injury and the consequences thereof, resulting in particular from: - excessive wear; - inappropriate use; - failure to comply with operating instructions, assembly instructions or maintenance instructions; - exceptional environmental, chemical, electrical or electrolytic influences. - faulty air or water seals or electrical connections. In any case the guarantee becomes null and void in the event of inappropriate servicing, use of nonrecommended parts, accessories or consumables, or modifications to the product carried out by third parties not authorized by Bien-Air Surgery SA. In case of dispute as so whether or not the defect exists, it shall be incumbent on the customer to prove the existence of the defect. Guarantee claims shall be taken into consideration only upon presentation, with the product, of a copy of the invoice or delivery slip on which should appear clearly the date of purchase and the product reference and serial number. 11.2.Governing law Swiss domestic law (“Code des obligations”) shall be applicable in addition to the general terms and particular agreements between the customer and Bien-Air Surgery SA. 11.3.Jurisdiction CH-2340 Le Noirmont, Switzerland.
Page 8/48
Set supplied:
REF 1500096 PMAM 1122-70 REF 1600207
REF 1500097
REF 1500098
X
PMAM 1122-95 REF 1600336
X
PMAM 1122-125 REF 1600337
X
REF 1000001
REF 1000009
X
X
X
X
X
X
PMAM 1121-70 REF 1600295
X
X
X
PMRM 1122-70 REF 1600206
X
X
X
PMRM 1124 REF 1600612
X
REF 1500003
REF 1500552
X
X
Page 44/48
EN
FR
DE
ES
IT
NL
STERILISATION TRAY
PANIER DE STERILISATION
STERILISATIONSKORB
CESTA DE ESTERILIZACIÓN
CESTELLO DI STERILIZZAZIONE
STERILISATIETRAY
BUR HOLDER AUTOCLAVABLE
PORTE-FRAISE STERILISABLE
STERILISIERBARE HALTERUNG
FRESERO ESTERILIZABLE
PORTA-FRESE AUTOCLAVABILE
BOORHOUDER AUTOCLAVEERBAAR
REF 1600477
REF 1600306
REF 1600064 / Lubrifluid (6 pcs per box)
Page 45/48
Page 46/48
Page 47/48
Bien-Air Surgery SA Rue de l’Ouest 2b CH-2340 Le Noirmont Switzerland Tél.+41(0)32 344 64 40 Fax +41(0)32 344 64 45 surgery@bienair.com Bien-Air Deutschland GmbH Surgery Jechtinger Strasse 11 79111 Freiburg, Deutschland Tel.+49 (0)761 45 57 40 Fax +49 (0)761 47 47 28 ba-d@bienair.com Bien-Air España S.A.U Surgery Entença, 169 Bajos 08029 Barcelona, España Tel. (+34) 934 25 30 40 Fax (+34) 934 23 98 60 ba-e@bienair.com
Bien-Air USA, Inc. Medical Technologies 5 Corporate Park Suite 160 Irvine, CA 92606, USA Phone 1-800-433-BIEN Phone 949-477-6050 Fax 949-477-6051 Ba-usa@bienair.com Bien-Air France Sarl Surgery 19-21, rue du 8 Mai 1945 94113 Arcueil, France Tel. +33 (0)1 49 08 02 60 Fax +33 (0)1 48 64 86 58 ba-f@bienair.com Bien-Air Italia s.r.l. Surgery Via Vaina 3 20122 Milano, Italia Tel. +39 (02) 58 32 12 51/52/53 Fax +39 (02) 58 32 12 53 Ba-i@bienair.com
Bien-Air UK Limited Surgery Arundel House Unit 1 - Ground Floor Amberley Court, Whitworth Road Crawley, West Sussex RH11 7XL, England Tel. +44 (0) 1293 550 200 Fax +44 (0) 1293 520 481 ba-uk@bienair.com Bien-Air Asia Ltd. Surgery Nishi-Ikebukuro Daiichi-Seimei Bldg. 10F 2-40-12 Ikebukuro, Toshimaku Tokyo, 171-0014, Japan
Tel.+81 (3) 5954-7661 Fax +81 (3) 5954-7660 ba-asia@bienair.com
Bien-Air on Internet: www.bienair.com = Sales = Repair
Page 48/48