Technical Manual
65 Pages
Preview
Page 1
EP // External Devices // Technical Manual
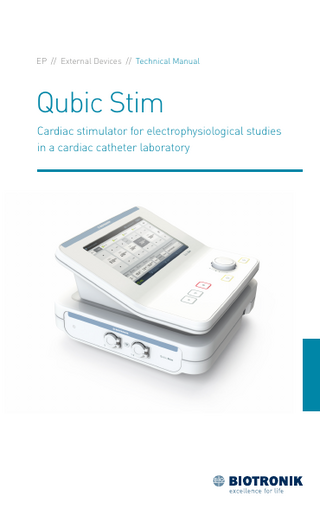
Qubic Stim Cardiac stimulator for electrophysiological studies in a cardiac catheter laboratory
399159--D_GA_QubicStim_en_Cover.indd 1
10.06.2017 16:34:06
399159--D_GA_QubicStim_en.fm Page 1 Thursday, June 15, 2017 4:46 PM
Table of Contents
1
Table of Contents Table of Contents
Introduction...2 About the Device...2 About this Technical Manual...3 Safety during Use...4 Required Expertise...4 Contraindications...4 Electromagnetic Interference...5 General Safety Information...6 Operating Conditions...8 Maintenance, Care and Disposal...10 Device Handling...13 Device Overview...13 Setting up the Device...18 Connections and Cables...19 Switching On and Off...24 Using the Software...25 Interface Concept...25 PES View...28 High-Rate View...37 SNRT View...39 Settings View...41 Appendix...45 Technical Data...45 Parameter Values...48 System Notifications...50 Accessories...53 Electromagnetic Compatibility According to EN 60601-1-2:2007...54 Legend for the Label...59 Directories...60 Index...60
399159--D
399159--D_GA_QubicStim_en.fm Page 2 Thursday, June 15, 2017 4:46 PM
2
Introduction
1
Introduction Introduction1397883Technical ManualQubic Stim
About the Device General description
Qubic Stim is a cardiac stimulator for electrophysiological studies in a cardiac catheter laboratory. The catheters, instruments, and the required accessories are used to carry out electrophysiological studies and to diagnose, among others, the Wolff-Parkinson-White syndrome, AV nodal reentrant tachycardia, and focal atrial and ventricular tachycardia.
Intended medical use
The purpose of Qubic Stim is diagnostic electrical stimulation of the heart such as induction and termination of tachyarrhythmia, refractory measurements and measurements of the electrical conduction system. The Qubic Stim stimulator has the following primary functions: Programmed extra stimulus (PES) High rate stimulation (burst) Measuring the sinus node recovery time (SNRT)
Patient group
Qubic Stim is indicated for all patients subjected to an electrophysiological study. Thus, the medical indication for the device is equivalent to the medical indication for electrophysiological studies with or without catheter ablation.
399159--D_GA_QubicStim_en.fm Page 3 Thursday, June 15, 2017 4:46 PM
Introduction
3
About this Technical Manual Objective
This technical manual provides all the safety information required to use the device. The following topics are covered in this manual: Device startup Device handling Using the software
Target group
This technical manual is intended for cardiologists, electrophysiologists and cardiac surgeons possessing knowledge in the following areas: Catheterization procedures Intracardiac stimulation and cardiac conduction systems This technical manual is also intended for clinical and technical assistants possessing expertise in handling devices in cardiac catheter laboratories. Additional required expertise is: Medical expertise: – Basic medical knowledge of the examination method employed Technical expertise: – Ability to work with a PC – Ability to use software-controlled medical devices
Other technical manuals
The following additional technical manuals must be followed to ensure the safe and correct use of the device: Technical manuals for other system components not supplied with the Qubic Stim (e.g. lab monitoring system) Technical manuals for the intended catheters, patient cables, and adapters Technical manuals for other designated accessories
399159--D_GA_QubicStim_en.fm Page 4 Thursday, June 15, 2017 4:46 PM
4
Safety during Use
2
Safety during Use Safety during Use2397883Technical ManualQubic Stim
Required Expertise Required expertise
Qubic Stim is intended for use by cardiologists, electrophysiologists, heart surgeons, and clinical and technical assistants specialized in handling devices in cardiac catheter laboratories and trained in handling the Qubic Stim. In addition to having basic medical knowledge, the user must be thoroughly familiar with the electrophysiology of the heart, catheterization procedures, and the method of ablating the intracardiac stimulation and conduction system. Only trained and qualified medical personnel with this knowledge can properly operate the device.
Contraindications Contraindications
Qubic Stim is not designed for permanent and nonmonitored application as an external pacemaker.
399159--D_GA_QubicStim_en.fm Page 5 Thursday, June 15, 2017 4:46 PM
Safety during Use
5
Electromagnetic Interference Possible electromagnetic interference
The device is protected from electromagnetic interference and electrostatic discharges. Also, the emitted interference has been reduced to a minimum. Thus the device conforms to the requirements of EN 606011-2 (in its current version at the time of delivery). However, strong electromagnetic interferences that occur in the close vicinity of electrical motors, power cables, PCs, monitors, or other – possibly defective – electrical devices may compromise the function of the device in certain cases. This kind of device malfunction should be considered as a possible cause if the following is observed: The device detects intrinsic cardiac events that cannot be seen in the ECG. The device displays other inexplicable functions. In the case of disruptions resulting from electromagnetic interference (EMI) that exceed certain limits, the Qubic Stim switches to the Fixed pacing mode. Pacing is performed with the set pacing rate. Correct operation of the device can be restored by the following methods: Switch off the malfunctioning electronic device. Remove the source of interference from the device. Switch the device on and off or break the electrical connection between the device and the source of the interference if this can be done safely. If the interference continues, contact BIOTRONIK immediately. Note: If accessories other than those specified by BIOTRONIK are used, increased interference or lower resistance to interference can be expected. Note: If accessories specified by BIOTRONIK are used on other devices, increased interference or lower resistance to interference can be expected. Note: Radio communication devices can interfere with how the device functions.
399159--D_GA_QubicStim_en.fm Page 6 Thursday, June 15, 2017 4:46 PM
6
Safety during Use
General Safety Information Risks of improper handling
Disregarding the safety warnings can endanger the patient, the staff and the equipment. Note: Failure to observe the safety warnings voids all damage claims and manufacturer liability. The following dangers may arise in the event of improper use: Failure of important device functions Personal endangerment due to electrical impact
Changes not permitted
Only the manufacturer or a party expressly authorized by BIOTRONIK may perform corrective maintenance, enhancements or modifications to the device.
Replacement parts and accessories
Only use accessories authorized by BIOTRONIK. Using any other parts voids liability for any consequences, as well as the product guarantee and warranty.
Defects
Do not use defective or damaged devices.
Physician supervision
The device may only be used under the constant supervision of a physician. The patient must be monitored at all times using an external surface ECG with rate control.
Patient observation
Ensure that patients are individually observed over a suitable period of time in order to monitor the compatibility and effectiveness of the electrophysiological study.
Emergency equipment
During an examination, keep resuscitation equipment (e.g., cardiac defibrillator, external pacemaker) available and ready for use at all times in order to be able to immediately perform life-supporting measures in the event of an emergency.
Not a life-support system Liquids
Never use this device as a life-support system. Never use a damp or wet device. Protect the device from accidental ingression of fluids (e.g. infusion fluids). If the device becomes wet, immediately unplug and stop using the device. Contact BIOTRONIK for testing and, if necessary, repair of the device.
399159--D_GA_QubicStim_en.fm Page 7 Thursday, June 15, 2017 4:46 PM
Safety during Use
7
Electrostatic potentials
Ensure that electrostatic potentials between medical staff and patients are balanced. Before handling the device, the electrostatic potential between the physician or medical staff and the patient must be balanced by touching the patient at a point as far away from the catheters or leads as possible.
Leakage currents
Avoid leakage currents between all connected devices. Such leakage currents can cause lethal arrhythmias. Potential equalization cables must be attached to all connected components, if present. Before initial commissioning, check and document all device combinations. National and international directives concerning the use of electromedical devices also apply to patient cables.
Sensitivity
A higher sensitivity level as set by selecting a lower sensitivity value will lead to more frequent interference from electromagnetic fields. Low sensitivity values should only be set if absolutely necessary for medical reasons.
Touching contacts on cables and catheters
Do not touch the contacts on the patient cable or on the catheter. The device has electrical contact with the patient's heart and blood via the implanted catheters. Touching the contacts on the patient cable or catheter could expose the patient's heart to dangerous electrical currents.
Defibrillation
When connected with the approved patient cable, the device is defibrillation protected. However, damage cannot be ruled out in all circumstances. Check all functions of the device, following a defibrillation. During defibrillation, do not touch the patient, the device the patient is connected to, or the attached accessories. Otherwise, there is a danger that you may suffer an electrical shock.
Risk of infection
Contaminated devices can lead to infection. Clean and disinfect the device on a regular basis. Observe the cleaning instructions for all other system components.
399159--D_GA_QubicStim_en.fm Page 8 Thursday, June 15, 2017 4:46 PM
8
Safety during Use
Operating Conditions Shipping and storage
If the package is damaged, please contact BIOTRONIK immediately. Do not put the device into operation. The ambient conditions for shipping and storage are:
Operating conditions
Temperature
+0°C ... +50°C
Relative humidity
30% ... 75%, no condensation
Atmospheric pressure
700 ... 1060 hPa
Note: After transporting the equipment from a cold to a warm area, condensation may form, particularly on metal parts of the device, and damage the electronics. After transport, wait approximately 2 h until the device has reached room temperature and the condensation has dried up before using the system. Only operate the device in rooms that fulfill the following conditions: No danger of explosion Suitable for medical purposes Class I power outlet with protective conductor connection Place the device in a position protected from spray water. Place the device on a flat, dry surface. Place the device in a position where it cannot slip, even with cables connected, and cannot be touched by the patient. The device cannot be sterilized. Set up the device in the sterile field using only a sterile cover (accessory).
The ambient conditions for operation are: Temperature
+10°C ... +40°C
Relative humidity
30% ... 75%, no condensation
Atmospheric pressure
700 ... 1060 hPa
Operation at altitudes
Up to 3000 m
399159--D_GA_QubicStim_en.fm Page 9 Thursday, June 15, 2017 4:46 PM
Safety during Use
Power supply
9
The device is operated via the AC voltage (100 to 240 V at 50 or 60 Hz) of a room used for medical purposes. CAUTION
!
Possibility of electric shock To avoid the risk of electric shock, connect the device only to a power supply fitted with a PE conductor. The electrical port must fulfill the following conditions: The power outlet fulfills at least the requirements of IEC 60364-7-710:2002 group 1. The cable feeds directly into a permanently installed socket. No portable multiple socket outlets should be used. When used in combination with other devices, no portable multiple socket outlets should be used. Only power cords which are suitable for medical devices can be used, such as power cords from BIOTRONIK or equivalent power cords labeled H05VV 3 x 0.75 mm, H05VV 3 x 1 mm, or SJT AWG18. The control unit is supplied with power via the associated VK-118 ethernet cable (max. 30 m) of the Stim Unit. To disconnect the Qubic Stim (consisting of the control unit and the Stim Unit) from the power supply, unplug the Stim Unit.
Cable and plug connections
Replace any cable that shows even slight damage. Lay all cables between the patient and the device, as well as within the measuring apparatus, in such a way that they pose no danger of tripping and that any tensile forces that may occur can be safely buffered. Ensure that the contacts of all connector ports and connectors are clean. Soiled contacts can lead to signal distortions, and thus to false diagnoses. Ensure that there is no condensation on the plugs or in the connector ports. If condensation is present, dry it before use. Do not force the plugs into the connector ports. Do not pull on the cable when disconnecting the plugs. Rather, release the lock on the plug. All lead connections are non-interchangeable and encoded at the lead connectors.
399159--D_GA_QubicStim_en.fm Page 10 Thursday, June 15, 2017 4:46 PM
10
Safety during Use
Maintenance, Care and Disposal General information
Cleaning and disinfecting
Note: Note the following points before cleaning and disinfecting: Disconnect the power plug before cleaning and disinfecting the device surfaces. Let cleaning and disinfection agents evaporate before operating the device. Do not use any strong and abrasive cleaning agents or organic solvents such as ether or benzine, as they corrode the surface of the device.
Sterilization Test before each use
Use lint-free, soft cloths. Clean the housing with a damp cloth and mild soap solution or 70% isopropanol. Disinfect with alcohol-based agents such as Aerodesin 2000. Visually inspect the plugs and connector ports: make sure that the contacts for all plugs and ports of the devices and cables are clean and free of any type of dirt.
The device cannot be sterilized.
A short test of the device and the approved accessories should be performed prior to each use. This test consists of the following visual inspections and a simple function test: – Inspect the housing for mechanical damage, dents, loose parts, cracks, etc. – Inspect cables and connection areas to ensure proper insulation, no breaks, etc. – Inspect the labeling for legibility. – Perform a simple electrical function test by switching on the device. An internal function test is performed automatically. If no error message appears, then no errors were found and the device can be used. – Inspect the displays (e.g., display of characters and language).
399159--D_GA_QubicStim_en.fm Page 11 Thursday, June 15, 2017 4:46 PM
Safety during Use
11
Inspection
The inspection consists of the regular technical safety check according to medical device standards. This ensures the safety of the device. Inspections should be performed: – If malfunctions are suspected – Once a year The inspection can be performed by BIOTRONIK. The inspection must conform to the manufacturer's specifications. These are available upon request. The specifications list all necessary test steps and the necessary equipment. The instructions for performing the inspection are directed at people whose education, knowledge, and experience obtained through practical work provide the basis for proper execution.
Fuse replacement
The fuses are located above the power cord port in a fuse holder. Step
Action
1
Turn the device off and unplug the power cord.
2
To unlock the fuse holder, push the latches on the right and left inwards together.
3
Pull out the fuse holder.
4
Replace the old fuses with new ones of the same type.
5
Re-insert the fuse holder and ensure that it locks securely in place.
Note: Defective fuses may indicate a technical defect in the device. Conduct an inspection after changing fuses and before resuming operation of the device.
399159--D_GA_QubicStim_en.fm Page 12 Thursday, June 15, 2017 4:46 PM
12
Safety during Use
Disposal
This device contains materials that must be correctly disposed of in accordance with environmental protection regulations. The European Directive 2012/19/EU regarding waste electrical and electronic equipment (WEEE) applies. The symbol on the type plate – a crossed out garbage can – indicates that the device must be disposed of in accordance with the WEEE directive. The black bar indicates that the device was sold after the national implementation of the WEEE directive had been enforced locally. Return devices that are no longer in use to BIOTRONIK.
Disposal of cables
Note: Cables that are to be disposed of must be treated as medical waste, in accordance with environmental regulations, if they have been in contact with blood. Non-contaminated cables must be disposed of in accordance with the European Directive 2012/19/EU regarding waste electrical and electronic equipment (WEEE).
399159--D_GA_QubicStim_en.fm Page 13 Thursday, June 15, 2017 4:46 PM
Device Handling
3
13
Device Handling Device Handling3397883Technical ManualQubic Stim
Device Overview Front view of the Stim Unit
1 2 3
4 Explanation of items Item Description 1
2
On/off light indicator (LED) Lights up green when the device is switched on On/off key For switching the device on/off
3
Redel port 1-2 For connecting a patient cable for stimulation channels 1 and 2
4
Redel port 3 For connecting a patient cable for stimulation channel 3
399159--D_GA_QubicStim_en.fm Page 14 Thursday, June 15, 2017 4:46 PM
14
Device Handling
Rear view of the Stim Unit
5 6 7
Explanation of items Item Description 5
Power cord port and device fuse For connecting the power cord
6
Ethernet port 1 (not suitable for network connection) For connecting a Control Unit
7
Ethernet port 2 (not suitable for network connection) For connecting a Control Unit
399159--D_GA_QubicStim_en.fm Page 15 Thursday, June 15, 2017 4:46 PM
Device Handling
Front view of Control Unit
8 9 10 11
12 13 14 Explanation of items Item Description 8
Screen (touch screen) For operating the device
9
Start key For starting a PES sequence
10
Pause key For interrupting a PES sequence
11
Stop key For stopping a PES sequence
12
13
High-rate key on right For delivery a high-rate stimulation (for the left hand if operating the device one-handed) Control dial For setting values
14
High-rate key on left For delivery a high-rate stimulation (for the right hand, if operating the device one-handed)
15
399159--D_GA_QubicStim_en.fm Page 16 Thursday, June 15, 2017 4:46 PM
16
Device Handling
Rear view of the Control Unit
15 16 17
Explanation of items Item Description 15
HDMI port For connecting an external monitor
16
Ethernet port (not suitable for network connection) For connecting the Control Unit to the Stim Unit
17
USB port For connecting a USB stick without independent power supply
399159--D_GA_QubicStim_en.fm Page 17 Thursday, June 15, 2017 4:46 PM
Device Handling
Symbols on the device
Explanation of symbols Symbol
Description On/off key
USB port HDMI port Follow the instructions for use
Fuse Control Unit 1 or 2
Stim Unit
Store in a dry place Observe the technical manual Type CF applied part, defibrillation protected Start a PES sequence or an SNRT measurement Interrupt a PES sequence
Stop a PES sequence or SNRT measurement Deliver a high-rate stimulation
17
399159--D_GA_QubicStim_en.fm Page 18 Thursday, June 15, 2017 4:46 PM
18
Device Handling
Setting up the Device General
CAUTION
!
Functional impairment due to external damage Mechanical impact can permanently impair the function of an unpackaged system even from a height of 5 cm (1.97") or greater. Do not use if the device or the package is visibly damaged. Contact BIOTRONIK for testing and, if necessary, repair of the device. Qubic Stim can be set up as a standalone device or in combination with a measuring station. The Stim Unit and control unit can be stacked as a unit or, if installed in 2 separate rooms, arranged a maximum distance of 30 m apart. The bottom of the control unit's housing contains 4 fixing holes to secure the control unit on a screen mount as per the VESA 75 standard (hole distance 75 x 75 mm and thread diameter M4). The device is not sterile and cannot be sterilized. Set up the device in the sterile field using only a sterile cover (accessory).
Setting up the device
Place the device in a position protected from spray water. Place the device on a flat, dry surface. Place the device in a position where it cannot slip, even with cables connected, and cannot be touched by the patient. The physician must not touch any plug connections such as USB ports and the patient at the same time.
399159--D_GA_QubicStim_en.fm Page 19 Thursday, June 15, 2017 4:46 PM
Device Handling
19
Connections and Cables Basic notes for cables and connections
Do not force the plugs into the connector ports. Do not pull on the cable when disconnecting the plugs. If necessary, release the lock on the plug.
Connecting the power cord
The power cord port on the device is designed to accept the power cord. The Stim Unit and control unit are supplied with power via the power cord. The power cord on the Stim Unit is located on the back side.
Before connecting, ensure that the power supply conditions are met (see Power supply, p. 9). Connect the power cord to the power cord port on the Stim Unit. To disconnect the Qubic Stim (consisting of the control unit and the Stim Unit) from the mains supply, unplug the Stim Unit. Connecting the control unit
The control unit is connected to the Stim Unit using the VK-118 Ethernet cable (max. 30 m). One control unit is provided standard. Depending on the software version (contact BIOTRONIK), you can connect up to 2 control units. In that case, one control unit operates in master mode and the other in slave mode. All settings may be made in master mode. The other control unit then operates in slave mode and merely displays the settings and values. You can switch between master and slave modes at any time through the user interface. The control units must be connected before switching on the device.