DePuy Synthes
10.5mm Monobloc Flexible Reamer - Standard
Depuy Synthes Monobloc Flexible Intramedullary Reamer Instrumentation System Instructions
189 Pages
Preview
Page 1
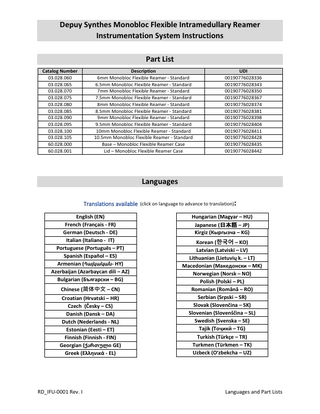
Depuy Synthes Monobloc Flexible Intramedullary Reamer Instrumentation System Instructions Part List Catalog Number 03.028.060 03.028.065 03.028.070 03.028.075 03.028.080 03.028.085 03.028.090 03.028.095 03.028.100 03.028.105 60.028.000 60.028.001
Description 6mm Monobloc Flexible Reamer - Standard 6.5mm Monobloc Flexible Reamer - Standard 7mm Monobloc Flexible Reamer - Standard 7.5mm Monobloc Flexible Reamer - Standard 8mm Monobloc Flexible Reamer - Standard 8.5mm Monobloc Flexible Reamer - Standard 9mm Monobloc Flexible Reamer - Standard 9.5mm Monobloc Flexible Reamer - Standard 10mm Monobloc Flexible Reamer - Standard 10.5mm Monobloc Flexible Reamer - Standard Base – Monobloc Flexible Reamer Case Lid – Monobloc Flexible Reamer Case
UDI 00190776028336 00190776028343 00190776028350 00190776028367 00190776028374 00190776028381 00190776028398 00190776028404 00190776028411 00190776028428 00190776028435 00190776028442
Languages Translations available (click on language to advance to translation): English (EN) French (Français - FR) German (Deutsch - DE) Italian (Italiano - IT) Portuguese (Português – PT) Spanish (Español – ES) Armenian (Հայկական- HY) Azerbaijan (Azərbaycan dili – AZ) Bulgarian (Български – BG) Chinese (简体中文 – CN) Croatian (Hrvatski – HR) Czech (Česky – CS) Danish (Dansk – DA) Dutch (Nederlands - NL) Estonian (Eesti – ET) Finnish (Finnish - FIN) Georgian (ქართული GE) Greek (Ελληνικά - EL)
RD_IFU-0001 Rev. I
Hungarian (Magyar – HU) Japanese (日本語 – JP) Kirgiz (Кыргызча – KG) Korean (한국어 – KO) Latvian (Latviski – LV) Lithuanian (Lietuvių k. – LT) Macedonian (Македонски – MK) Norwegian (Norsk – NO) Polish (Polski – PL) Romanian (Română – RO) Serbian (Srpski – SR) Slovak (Slovenčina – SK) Slovenian (Slovenščina – SL) Swedish (Svenska – SE) Tajik (Тоҷикӣ – TG) Turkish (Türkçe – TR) Turkmen (Türkmen – TK) Uzbeck (Oʻzbekcha – UZ)
Languages and Part Lists
English EN Monobloc Flexible Intramedullary Reamer Instrumentation Instructions INTENDED USE •
The Monobloc Flexible Intramedullary Reamers are intended to ream an intramedullary bone canal in preparation for insertion of implants (e.g. intramedullary nails or stems).
INTENDED USER PROFILE • •
Surgical procedures should be performed only by persons having adequate training and familiarity with surgical techniques including progressive reaming procedures. Consult medical literature relative to techniques, complications and hazards prior to performance of any surgical procedure. Before using the product, all instructions regarding its safety features must be read carefully.
DEVICE DESCRIPTION • • • • •
Surgical instruments comprising monobloc constructs generally composed of medical grade stainless steels. o Drill Power compatibility by a Modified Trinkle attachment or Jacobs Chuck. o Ball-tip guidewire/reaming rod compatibility: Ø2.5GW Only. Instrument case and trays may consist of different materials including stainless steels, aluminum and silicone mats. Instruments are supplied NON-STERILE and must be inspected, cleaned and sterilized before each use. Devices are critical and require terminal sterilization per FDA guidelines and the Spaulding Classification scheme. Devices are not implantable.
WARNINGS • • • • • • • • • • • •
Avalign recommends thorough manual and automated cleaning of medical devices prior to sterilization. Automated methods alone may not adequately clean devices. Devices must be dry before being packaged for sterilization. Devices should be reprocessed as soon as possible following use. Instruments must be cleaned separately from cases and trays. Flexible devices contain challenging features and require special attention during cleaning. Repeated flexing or overflexing of devices could have adverse effects on the fatigue properties and lifetime of the device. All cleaning agent solutions should be replaced frequently before becoming heavily soiled. Prior to cleaning, sterilization and use, remove all protective caps carefully. All instruments should be inspected to ensure proper function and condition. Do not use instruments if they do not perform satisfactorily. The sterilization methods described have been validated with the devices in predetermined placement locations per the case design. Areas intended for specific devices shall contain only those devices. Blunt and/or damaged reamer heads increase intramedullary pressure and temperature when reaming and should be inspected and discarded prior to clinical use. Devices must be used on a Drill Torque setting. Use on a Ream Torque setting may cause premature failures. Devices must be used over a Ø2.5mm ball-tip guidewire to secure the connection between the reamer head and flexible shaft and to support standard retrieval strategies from the bone canal. Risk of damage – The surgical instrument is a precision device. Careful handling is important for accurate functioning of the product. Improper external handling can cause product malfunction. Use caution when handling sharp instruments to avoid injury. If a device is/was used in a patient with, or suspected of having Creutzfeldt-Jakob Disease (CJD), the device cannot be reused and must be destroyed due to the inability to reprocess or sterilize to eliminate the risk of cross-contamination.
CAUTION
Federal U.S. Law restricts this device to sale, distribution, and use, by, or on order of a physician. LIMITATIONS ON REPROCESSING Repeated processing has minimal effect on these instruments. End of life is normally determined by wear and damage due to use. DISCLAIMER It is the responsibility of the reprocessor to ensure reprocessing is performed using equipment, materials and personnel in the reprocessing facility and achieves the desired result. This requires validation and routine monitoring of the process. Any deviation by the reprocessor from the instructions provided must be properly evaluated for effectiveness and potential adverse consequences.
RD_IFU-0001 Rev. I
Page 1 of 5
Reprocessing Instructions TOOLS AND ACCESSORIES Water Cleaning Agents
Accessories
Equipment
Cold Tap Water (< 20°C / 68°F) Hot Tap Water (> 40°C / 104°F) Deionized (DI) or Reverse Osmosis (RO) Water (ambient) Enzymatic Cleaner pH 6.0-8.0 i.e. MetriZyme, EndoZime, Enzol Neutral Detergent pH 6.0-8.0 i.e. Liqui-nox, Valsure Assorted Sizes of Brushes and/or Pipe Cleaners with Nylon Bristles Sterile Syringes or equivalent Absorbent, Low Lint Disposable Cloths or equivalent Soaking Pans Hydrogen Peroxide Medical Compressed Air Ultrasonic Cleaner Automated Washer
POINT-OF-USE AND CONTAINMENT 1) 2) 3)
Follow health care facility point of use practices. Keep devices moist after use to prevent soil from drying and remove excess soil and debris from all lumens, surfaces, crevices, sliding mechanisms, hinged joints, flexible areas and all other hard-to-clean design features. Suction or flush lumens with a cleaning solution immediately after use. Follow universal precautions and contain devices in closed or covered containers for transport to central supply.
MANUAL CLEANING 4) 5) 6)
7) 8)
Disassemble all devices as warranted per manufacturer’s instructions. Rinse devices under cold running tap water for a minimum of 3 minutes while wiping off residual soil or debris. Actuate moveable mechanisms and flush all lumens, cracks and/or crevices while rinsing. If the device has flexible areas, bend or flex the shaft multiple directions while rotating to ensure adequate rinsing of all surfaces. Prepare an enzymatic cleaning solution per manufacturer’s instructions including dilution/concentration, water quality and temperature. Immerse devices and soak for a minimum of 10 minutes. While in the solution, use a soft, bristle brush to remove all traces of blood and debris from the device, paying close attention to threads, crevices, seams, and any hard to reach areas. a) If the device has sliding mechanisms or hinged joints, actuate the device while scrubbing to remove trapped soil. b) If the device contains a lumen, use a tight-fitting nylon brush or pipe cleaner while pushing in and out with a twisting motion to facilitate removal of debris; ensure the full diameter and depth of the lumen is accessed. Flush the lumen, three times minimum, with a syringe containing a minimum solution of 60mL. c) If the device has flexible areas, bend or flex the shaft multiple directions in the solution and use a scrub brush and twisting action to clean all surfaces while rotating the part. Remove devices and rinse/agitate in cold tap water for a minimum of 3 minutes. Actuate moveable mechanisms and flush all lumens, cracks and/or crevices while rinsing. If the device has flexible areas, bend or flex the shaft multiple directions while rotating to ensure adequate rinsing of all surfaces. Prepare a neutral detergent cleaning solution per manufacturer’s instructions including dilution/concentration, water quality and temperature. Immerse devices and soak for a minimum of 5 minutes. While in the solution, use a soft, bristle brush to remove all traces of blood and debris from the device, paying close attention to threads, crevices, seams, and any hard to reach areas. a) If the device has sliding mechanisms or hinged joints, actuate the device while scrubbing to remove trapped soil. b) If the device contains a lumen, use a tight-fitting nylon brush or pipe cleaner while pushing in and out with a twisting motion to facilitate removal of debris; ensure the full diameter and depth of the lumen is accessed. Flush the lumen, three times minimum, with a syringe containing a minimum solution of 60mL. c) If the device has flexible areas, bend or flex the shaft multiple directions in the solution and use a scrub brush and twisting action to clean all surfaces while rotating the part.
9)
Remove devices and rinse/agitate in cold tap water for a minimum of 3 minutes. Actuate moveable mechanisms and flush all lumens, cracks and/or crevices while rinsing. If the device has flexible areas, bend or flex the shaft slightly in multiple directions while rotating to ensure adequate rinsing of all surfaces. 10) Prepare an enzymatic cleaning solution using hot water per manufacturer’s recommendations in an ultrasonic unit. Sonicate the devices for a minimum of 15 minutes using a minimum frequency of 40 kHz. It is recommended to use an ultrasonic unit with flushing attachments. Devices with lumens should be flushed with cleaning solution under the surface of the solution to ensure adequate perfusion of channels. 11) Remove devices and rinse/agitate in ambient DI/RO water for a minimum of 4 minutes. Actuate moveable mechanisms and flush all lumens, cracks and/or crevices while rinsing. If the device has flexible areas, bend or flex the shaft multiple directions while rotating for a minimum of 2 minutes to ensure adequate rinsing of all surfaces.
RD_IFU-0001 Rev. I
Page 2 of 5
12) Dry the device using an absorbent cloth. Dry any internal areas with filtered, compressed air. 13) Visually inspect the device for soil under magnification including all actuating mechanisms, cracks, crevices, and lumens. If not visibly clean, repeat steps 4-13. 14) Submerge device in 2-3% hydrogen peroxide. The appearance of bubbles confirms the presence of hemoglobin. Repeat steps 5-14 if bubbles appear. Adequately rinse device with DI/RO water. AUTOMATED CLEANING Note: All devices must be manually pre-cleaned prior to any automated cleaning process, follow steps 1-9. Steps 10-14 are optional but advised. 15) Transfer the devices to an automatic washer/disinfector for processing per the below minimum parameters. Detergent Type & Concentration Pre-wash 1 02:00 Cold Tap Water N/A Enzyme Wash 02:00 Hot Tap Water Enzyme Detergent Wash 1 02:00 63°C / 146°F Neutral Detergent Rinse 1 02:00 Hot Tap Water N/A Purified Water Rinse 02:00 63°C / 146°F N/A Drying 07:00 115°C / 240°F N/A 16) Dry excess moisture using an absorbent cloth. Dry any internal areas with filtered, compressed air. 17) Visually inspect the device for soil under magnification including all actuating mechanisms, cracks, crevices and lumens. If not visibly clean, repeat steps 4-9, 15-17. 18) Submerge device in 2-3% hydrogen peroxide. The appearance of bubbles confirms the presence of hemoglobin. Repeat steps 5-9, 15-18 if bubbles appear. Adequately rinse device with DI/RO water. Phase
Time (minutes)
Temperature
DISINFECTION • • • •
Devices must be terminally sterilized (See § Sterilization). Avalign instruments are compatible with washer/disinfector time-temperature profiles for thermal disinfection per ISO 15883. Load the devices in the washer-disinfector according to the manufacturer’s instructions, ensuring that the devices and lumens can drain freely. The following automated cycles are examples of validated cycles: Phase Thermal Disinfection Thermal Disinfection
Recirculation Time (min.) 1 5
Water Temperature >90°C (194°F) >90°C (194°F)
Water Type RI/DO Water RI/DO Water
INSPECTION AND FUNCTIONAL TESTING • • •
Visually inspect devices for damage or wear. Instruments with broken, cracked, chipped or worn parts, or tarnished surfaces should not be used, but should be replaced immediately. Check that reamer cutting edges are smooth and continuous, free from large cracks or chips that may impair cutting performance. Verify device interfaces with power drill without complications.
PACKAGING • • • •
Only FDA cleared sterilization packaging materials should be used by the end user when packaging the devices. The end user should consult ANSI/AAMI ST ST79 or ISO 17765-1 for additional information on steam sterilization. Sterilization Wrap o Cases may be wrapped in a standard, medical grade sterilization wrap using the AAMI double wrap method or equivalent. Rigid Sterilization Container o For information regarding rigid sterilization containers, please refer to appropriate instructions for use provided by the container manufacturer or contact the manufacturer directly for guidance.
STERILIZATION Sterilize with steam. The following are minimum cycles required for steam sterilization of Avalign devices:
Double Wrapped Instrument Case: Cycle Type Prevacuum Prevacuum RD_IFU-0001 Rev. I
Temperature 132°C (270°F) 134°C (273°F)
Exposure Time 4 minutes 3 minutes
Pulses 4 4
Drying Time 20 minutes 30 minutes Page 3 of 5
Single Instrument Case Enclosed in Rigid Sterilization Container: Cycle Type Prevacuum • •
•
•
•
Temperature 132°C (270°F)
Exposure Time 4 minutes
Pulses 4
Drying Time 30 minutes
The operating instructions and guidelines for maximum load configuration of the sterilizer manufacturer should be followed explicitly. The sterilizer must be properly installed, maintained, and calibrated. Time and temperature parameters required for sterilization vary according to type of sterilizer, cycle design, and packaging material. It is critical that process parameters be validated for each facility’s individual type of sterilization equipment and product load configuration. Avalign devices were validated under laboratory conditions using the biological indicator (BI) overkill method to achieve a sterility assurance level (SAL) of 10-6 in a double wrapped instrument case or a single instrument case enclosed by the appropriate rigid sterilization container. Only steam sterilization cycles have been validated for use and have been shown to be compatible with the device design. A facility may choose to use different steam sterilization cycles other than the cycle suggested if the facility has properly validated the cycle to ensure adequate steam penetration and contact with the devices for sterilization. Note: rigid sterilization containers cannot be used in gravity steam cycles. Water droplets and visible signs of moisture on sterile packaging/wrap or the tape used to secure it may compromise the sterility of the processed loads or be indicative of a sterilization process failure. Visually check outside wrap for dryness. If there are water droplets for visible moisture observed the pack or instrument tray is considered unacceptable. Repackaging and re-sterilize the packages with visible signs of moisture.
STORAGE • •
After sterilization, instruments should remain in sterilization packaging and be stored in a clean, dry cabinet or storage case. Care should be taken when handling devices to avoid damaging the sterile barrier.
MAINTENANCE • •
Discard damaged, worn or non-functional devices. Reamer heads cannot be resharpened.
WARRANTY • •
All products are guaranteed to be free from defects in material and workmanship at the time of shipping. Avalign instruments are reusable and meet AAMI standards for sterilization. All our products are designed and manufactured to meet the highest quality standards. We cannot accept liability for failure of products which have been modified in any way from their original design.
CONTACT • Notice to Patient and User: Any serious incident that has occurred in relation to the medical devices should be reported to the manufacturer and the competent authority of the EU Member State in which the user and/or patient is established. Manufactured by: Avalign Technologies 8727 Clinton Park Drive Fort Wayne, IN 46825 1-877-289-1096 www.avalign.com product.questions@avalign.com
Authorized Representative Reamer Instruments: Instrumed GmbH (dba Avalign German Specialty Instruments) Unter Buchsteig 3 78 532 Tuttlingen, Germany
2797
RD_IFU-0001 Rev. I
Authorized Representative Case and Tray: Emergo Europe, Prinsessegracht 20, 2514 AP The Hague, The Netherlands
Distributed in US By: Synthes USA, LLC 1101 Synthes Avenue Monument, CO 80132 Distributed OUS By: Synthes GMBH Eimattstrasse 3 4436 Oberdorf Switzerland Page 4 of 5
Label Glossary Symbol
Title and Translations Manufacturer and Date of Manufacture
RD_IFU-0001 Rev. I
Symbol
Title and Translations Consult Instructions for Use
Authorized Representative in the European Community
Caution
Lot Number / Batch Code
Federal Law (USA) restricts this device to sale by or on the order of a physician
Catalogue Number
Medical Device
Page 5 of 5