MO Milling
Nitinol Guide Wire
Nitinol Guidewire Product Intended Use , Requirements and Specifications
2 Pages
Preview
Page 1
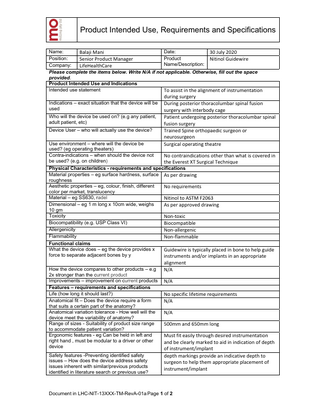
Product Intended Use, Requirements and Specifications Date: Name: Balaji Mani 30 July 2020 Product Position: Senior Product Manager Nitinol Guidewire Name/Description: Company: LifeHealthCare Please complete the items below. Write N/A if not applicable. Otherwise, fill out the space provided. Product Intended Use and Indications Intended use statement To assist in the alignment of instrumentation Indications – exact situation that the device will be used Who will the device be used on? (e.g any patient, adult patient, etc) Device User – who will actually use the device? Use environment – where will the device be used? (eg operating theaters) Contra-indications – when should the device not be used? (e.g. on children)
during surgery During posterior thoracolumbar spinal fusion surgery with interbody cage Patient undergoing posterior thoracolumbar spinal fusion surgery Trained Spine orthopaedic surgeon or neurosurgeon Surgical operating theatre No contraindications other than what is covered in the Everest XT Surgical Technique
Physical Characteristics - requirements and specifications Material properties – eg surface hardness, surface As per drawing roughness Aesthetic properties – eg, colour, finish, different No requirements color per market, translucency Material – eg SS630, radel Nitinol to ASTM F2063 Dimensional – eg 1 m long x 10cm wide, weighs As per approved drawing 10 gm Toxicity Non-toxic Biocompatibility (e.g. USP Class VI) Biocompatible Allergenicity Non-allergenic Flammability Non-flammable Functional claims What the device does – eg the device provides x Guidewire is typically placed in bone to help guide force to separate adjacent bones by y instruments and/or implants in an appropriate How the device compares to other products – e.g 2x stronger than the current product Improvements – improvement on current products Features – requirements and specifications Life (how long it should last?) Anatomical fit – Does the device require a form that suits a certain part of the anatomy? Anatomical variation tolerance - How well will the device meet the variability of anatomy? Range of sizes - Suitability of product size range to accommodate patient variation? Ergonomic features - eg Can be held in left and right hand , must be modular to a driver or other device Safety features -Preventing identified safety issues – How does the device address safety issues inherent with similar/previous products identified in literature search or previous use?
alignment N/A N/A No specific lifetime requirements N/A N/A 500mm and 650mm long Must fit easily through desired instrumentation and be clearly marked to aid in indication of depth of instrument/implant depth markings provide an indicative depth to surgeon to help them appropriate placement of instrument/implant
Document in LHC-NIT-13XXX-TM-RevA-01a Page 1 of 2
Product Intended Use, Requirements and Specifications Safety features- Control measures identified in Risk Assessment Reliability - In what ways will the device achieve reliability? End of device life - How does user know when products life has ended? Interface Requirements and Specifications Associated tools, equipment Sizing Clinician requirements and specifications Procedural timeframe , how long will the device be used , eg less than 30 minutes Qualifications/experience requirements for procedure Sterility (e.g. non-sterile, steam autoclaveable at xx degrees for yy minutes)
Other Requirements and Specifications Manufacturing , eg must be manufactured under ISO 13485 QMS Cost/Economic (e.g. must be less than $xx) Regulatory , eg to be registered with TGA as a class 1 product Packaging (e.g. biocompatible, sealed)
Verification of critical dimensions identified in 2D drawing Can be re-used provided not damaged If the device shows signs of wear and tear it will need to be replaced or refurbished Instrument/implant with sufficiently sized cannula As per drawing Less than 60 minutes Trained Spine orthopaedic surgeon or neurosurgeon Autoclavable - Flash Steam Cycle: 132°C (270°F), 15 minutes (Gravity Displacement Cycle), or minimum 3 minutes (Dynamic Air Removal (Prevacuum) Cycle ISO13485 QMS N/A TGA class 1 device Package in a sealed plastic bag and boxed in a carton suitable for courier transportation Label with part number, lot number
Labelling (e.g. with part number and suitable for courier transportation, complies with ISO15223-1 Training requirements (e.g. user to be trained on No requirements the instruction for use before using the device) Requirements and Specifications not covered above
None
Document in LHC-NIT-13XXX-TM-RevA-01a Page 2 of 2